Safety and efficacy of multipotent adult progenitor cells in acute respiratory distress syndrome (MUST-ARDS): a multicentre, randomised, double-blind, placebo-controlled phase 1/2 trial
- PMID: 34811567
- PMCID: PMC8608557
- DOI: 10.1007/s00134-021-06570-4
Safety and efficacy of multipotent adult progenitor cells in acute respiratory distress syndrome (MUST-ARDS): a multicentre, randomised, double-blind, placebo-controlled phase 1/2 trial
Abstract
Purpose: Bone marrow-derived, allogeneic, multipotent adult progenitor cells demonstrated safety and efficacy in preclinical models of acute respiratory distress syndrome (ARDS).
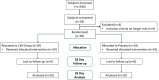
Methods: This phase 1/2 trial evaluated the safety and tolerability of intravenous multipotent adult progenitor cells in patients with moderate-to-severe ARDS in 12 UK and USA centres. Cohorts 1 and 2 were open-label, evaluating acute safety in three subjects receiving 300 or 900 million cells, respectively. Cohort 3 was a randomised, double-blind, placebo-controlled parallel trial infusing 900 million cells (n = 20) or placebo (n = 10) within 96 h of ARDS diagnosis. Primary outcomes were safety and tolerability. Secondary endpoints included clinical outcomes, quality of life (QoL) and plasma biomarkers.
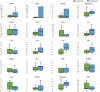
Results: No allergic or serious adverse reactions were associated with cell therapy in any cohort. At baseline, the cohort 3 cell group had less severe hypoxia. For cohort 3, 28-day mortality was 25% for cell vs. 45% for placebo recipients. Median 28-day free from intensive care unit (ICU) and ventilator-free days in the cell vs. placebo group were 12.5 (IQR 0,18.5) vs. 4.5 (IQR 0,16.8) and 18.5 (IQR 0,22) vs. 6.5 (IQR 0,18.3), respectively. A prospectively defined severe ARDS subpopulation (PaO2/FiO2 < 150 mmHg (20 kPa); n = 16) showed similar trends in mortality, ICU-free days and ventilator-free days favouring cell therapy. Cell recipients showed greater recovery of QoL through Day 365.
Conclusions: Multipotent adult progenitor cells were safe and well tolerated in ARDS. The clinical outcomes warrant larger trials to evaluate the therapeutic efficacy and optimal patient population.
Trial registration: ClinicalTrials.gov NCT02611609.
Keywords: Acute respiratory distress syndrome (ARDS); Multipotent adult progenitor cells (MAPC); Stem cells.
© 2021. Crown.
Conflict of interest statement
Two of the authors, EJ and AT, were employees of Athersys and one, GB, received a travel grant from Athersys to two meetings. No other Conflict of interest are declared
Figures
Similar articles
-
Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-AT): A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 6;22(1):9. doi: 10.1186/s13063-020-04964-1. Trials. 2021. PMID: 33407777 Free PMC article.
-
A randomised, double-blind, placebo-controlled, pilot trial of intravenous plasma purified alpha-1 antitrypsin for SARS-CoV-2-induced Acute Respiratory Distress Syndrome: a structured summary of a study protocol for a randomised, controlled trial.Trials. 2021 Apr 19;22(1):288. doi: 10.1186/s13063-021-05254-0. Trials. 2021. PMID: 33874981 Free PMC article.
-
Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet Neurol. 2017 May;16(5):360-368. doi: 10.1016/S1474-4422(17)30046-7. Epub 2017 Mar 17. Lancet Neurol. 2017. PMID: 28320635 Clinical Trial.
-
Immunonutrition for acute respiratory distress syndrome (ARDS) in adults.Cochrane Database Syst Rev. 2019 Jan 24;1(1):CD012041. doi: 10.1002/14651858.CD012041.pub2. Cochrane Database Syst Rev. 2019. PMID: 30677127 Free PMC article.
-
Endogenous and exogenous cell-based pathways for recovery from acute respiratory distress syndrome.Clin Chest Med. 2014 Dec;35(4):797-809. doi: 10.1016/j.ccm.2014.08.015. Epub 2014 Sep 24. Clin Chest Med. 2014. PMID: 25453426 Free PMC article. Review.
Cited by
-
Mesenchymal stem/stromal cell-based therapies for severe viral pneumonia: therapeutic potential and challenges.Intensive Care Med Exp. 2021 Dec 31;9(1):61. doi: 10.1186/s40635-021-00424-5. Intensive Care Med Exp. 2021. PMID: 34970706 Free PMC article. Review.
-
Cell-based Therapies for Acute Respiratory Distress Syndrome: Where Are We Now?Am J Respir Crit Care Med. 2024 Apr 1;209(7):789-797. doi: 10.1164/rccm.202311-2046CP. Am J Respir Crit Care Med. 2024. PMID: 38324017 Free PMC article.
-
Vascular leak in sepsis: physiological basis and potential therapeutic advances.Crit Care. 2024 Mar 23;28(1):97. doi: 10.1186/s13054-024-04875-6. Crit Care. 2024. PMID: 38521954 Free PMC article. Review.
-
Therapeutic impact of mesenchymal stem cells on idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation.Int J Hematol. 2025 Jun 9. doi: 10.1007/s12185-025-04013-0. Online ahead of print. Int J Hematol. 2025. PMID: 40489033
-
Safety, efficacy and biomarkers analysis of mesenchymal stromal cells therapy in ARDS: a systematic review and meta-analysis based on phase I and II RCTs.Stem Cell Res Ther. 2022 Jun 25;13(1):275. doi: 10.1186/s13287-022-02956-3. Stem Cell Res Ther. 2022. PMID: 35752865 Free PMC article.
References
-
- Group RC. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. - DOI
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

