Recent developments in the chiroptical properties of chiral plasmonic gold nanostructures: bioanalytical applications
- PMID: 34811580
- PMCID: PMC8608422
- DOI: 10.1007/s00604-021-05066-8
Recent developments in the chiroptical properties of chiral plasmonic gold nanostructures: bioanalytical applications
Abstract
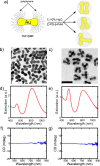
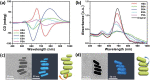
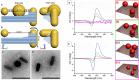
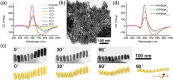
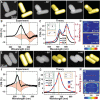
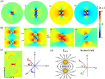
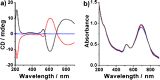
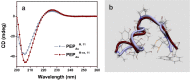
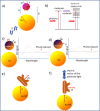
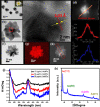
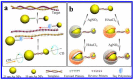
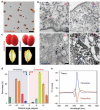
The presence of excess L-amino acid in the Murchison meteorite, circular polarization effect in the genesis of stars and existence of chirality in interstellar molecules contribute to the origin of life on earth. Chiral-sensitive techniques have been employed to untangle the secret of the symmetries of the universe, designing of effective secure drugs and investigation of chiral biomolecules. The relationship between light and chiral molecules was employed to probe and explore such molecules using spectroscopy techniques. The mutual interaction between electromagnetic spectrum and chirality of matter give rise to distinct optical response, which advances vital information contents in chiroptical spectroscopy. Chiral plasmonic gold nanoparticle exhibits distinctive circular dichroism peaks in broad wavelength range thereby crossing the limits of its characterization. The emergence of strong optical activity of gold nanosystem is related to its high polarizability, resulting in plasmonic and excitonic effects on incident photons. Inspired by the development of advanced chiral plasmonic nanomaterials and exploring its properties, this review gives an overview of various chiral gold nanostructures and the mechanism behind its chiroptical properties. Finally, we highlight the application of different chiral gold nanomaterials in the field of catalysis and medical applications with special emphasis to biosensing and biodetection.
Keywords: Chirality; Chiroptical properties; Circular dichroism; Gold nanostructures; Plasmonic nanoparticle; Surface plasmon resonance.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Austria, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Wu W, Hu W, Qian G, et al (2019) Mechanical design and multifunctional applications of chiral mechanical metamaterials: a review. Mater Des 180:107950.10.1016/j.matdes.2019.107950
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
