α-synuclein suppresses microglial autophagy and promotes neurodegeneration in a mouse model of Parkinson's disease
- PMID: 34811872
- PMCID: PMC8672776
- DOI: 10.1111/acel.13522
α-synuclein suppresses microglial autophagy and promotes neurodegeneration in a mouse model of Parkinson's disease
Abstract
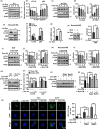
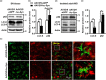
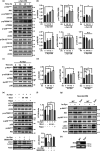
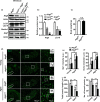
The cell-to-cell transfer of α-synuclein (α-Syn) greatly contributes to Parkinson's disease (PD) pathogenesis and underlies the spread of α-Syn pathology. During this process, extracellular α-Syn can activate microglia and neuroinflammation, which plays an important role in PD. However, the effect of extracellular α-Syn on microglia autophagy is poorly understood. In the present study, we reported that extracellular α-Syn inhibited the autophagy initiation, as indicated by LC3-II reduction and p62 protein elevation in BV2 and cultured primary microglia. The in vitro findings were verified in microglia-enriched population isolated from α-Syn-overexpressing mice induced by adeno-associated virus (AAV2/9)-encoded wildtype human α-Syn injection into the substantia nigra (SN). Mechanistically, α-Syn led to microglial autophagic impairment through activating toll-like receptor 4 (Tlr4) and its downstream p38 and Akt-mTOR signaling because Tlr4 knockout and inhibition of p38, Akt as well as mTOR prevented α-Syn-induced autophagy inhibition. Moreover, inhibition of Akt reversed the mTOR activation but failed to affect p38 phosphorylation triggered by α-Syn. Functionally, the in vivo evidence showed that lysozyme 2 Cre (Lyz2cre )-mediated depletion of autophagy-related gene 5 (Atg5) in microglia aggravated the neuroinflammation and dopaminergic neuron losses in the SN and exacerbated the locomotor deficit in α-Syn-overexpressing mice. Taken together, the results suggest that extracellular α-Syn, via Tlr4-dependent p38 and Akt-mTOR signaling cascades, disrupts microglial autophagy activity which synergistically contributes to neuroinflammation and PD development.
Keywords: Parkinson's disease; autophagy; microglia; neuroinflammation; α-synuclein.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
We have no potential conflict of interest to be disclosed.
Figures

References
-
- Bae, E. J. , Lee, H. J. , Rockenstein, E. , Ho, D. H. , Park, E. B. , Yang, N. Y. , Desplats, P. , Masliah, E. , & Lee, S. J. (2012). Antibody‐aided clearance of extracellular alpha‐synuclein prevents cell‐to‐cell aggregate transmission. Journal of Neuroscience, 32(39), 13454–13469. 10.1523/JNEUROSCI.1292-12.2012 - DOI - PMC - PubMed
-
- Cheng, J. , Liao, Y. , Dong, Y. , Hu, H. , Yang, N. , Kong, X. , Li, S. , Li, X. , Guo, J. , Qin, L. , Yu, J. , Ma, C. , Li, J. , Li, M. , Tang, B. , & Yuan, Z. (2020). Microglial autophagy defect causes Parkinson disease‐like symptoms by accelerating inflammasome activation in mice. Autophagy, 16(12), 2193–2205. 10.1080/15548627.2020.1719723 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

