Hide-and-seek: Neurotropic squamous cell carcinoma of the periorbital region - a series of five cases and review of the literature
- PMID: 34811913
- PMCID: PMC9299592
- DOI: 10.1111/ddg.14582
Hide-and-seek: Neurotropic squamous cell carcinoma of the periorbital region - a series of five cases and review of the literature
Abstract
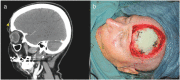
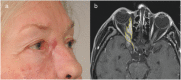
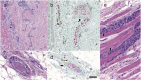
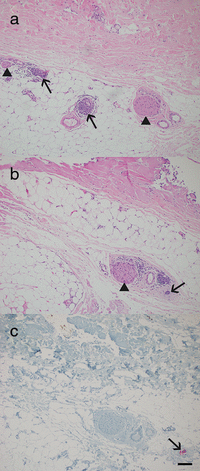
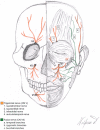
Squamous cell carcinoma is the second most common malignancy of the skin after basal cell carcinoma and mainly found in sun-exposed areas such as the face. This mostly locally destructive malignancy may show invasive growth and insidious mechanisms of dissemination such as perineural invasion. Periorbital squamous cell carcinoma is associated with perineural invasion in up to 14 % of cases. Specifically in this region, the proximity to cranial nerves and therefore the associated risk of progression to the central nervous system are associated with poor prognosis. The clinically concealed character of this entity often leads to a delay in diagnosis and consequently makes complete resection and reconstruction demanding. Careful clinical evaluation often hints at perineural invasion before obtaining histology. Aside from presenting five challenging cases, this work analyzes risk factors, clinical as well as histological features, and treatment options for periorbital squamous cell carcinoma with perineural invasion.
© 2021 The Authors. Journal der Deutschen Dermatologischen Gesellschaft published by John Wiley & Sons Ltd on behalf of Deutsche Dermatologische Gesellschaft.
Conflict of interest statement
None.
Figures


References
-
- Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer – the role of sunlight. Adv Exp Med Biol 2008; 624: 89–103. - PubMed
-
- Leiter U, Heppt MV, Steeb T et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma (cSCC) – short version, part 2: epidemiology, surgical and systemic treatment of cSCC, follow‐up, prevention and occupational disease. J Dtsch Dermatol Ges 2020; 18(4): 400–13. - PubMed
-
- Brantsch KD, Meisner C, Schönfisch B et al. Analysis of risk factors determining prognosis of cutaneous squamous‐cell carcinoma: a prospective study. Lancet Oncol 2008; 9(8): 713–20. - PubMed
-
- Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 1985; 94(4 Pt 1): 426–7. - PubMed
-
- Moore CE, Hoyt WF, North JB. Painful ophthalmoplegia following treated squamous carcinoma of the forehead. Orbital apex involvement from centripetal spread via the supraorbital nerve. Med J Aust 1976; 1(18): 657–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

