Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare through Human and Environmental Treatment
- PMID: 34811960
- PMCID: PMC8986592
- DOI: 10.1002/adhm.202101820
Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare through Human and Environmental Treatment
Abstract
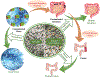
Humans are constantly exposed to exogenous chemicals throughout their life, which can lead to a multitude of negative health impacts. Advanced materials can play a key role in preventing or mitigating these impacts through a wide variety of applications. The tunable properties of hydrogels and hydrogel nanocomposites (e.g., swelling behavior, biocompatibility, stimuli responsiveness, functionality, etc.) have deemed them ideal platforms for removal of environmental contaminants, detoxification, and reduction of body burden from exogenous chemical exposures for prevention of disease initiation, and advanced treatment of chronic diseases, including cancer, diabetes, and cardiovascular disease. In this review, three main junctures where the use of hydrogel and hydrogel nanocomposite materials can intervene to positively impact human health are highlighted: 1) preventing exposures to environmental contaminants, 2) prophylactic treatments to prevent chronic disease initiation, and 3) treating chronic diseases after they have developed.
Keywords: chronic diseases; hydrogel nanocomposites; hydrogels; remediations; therapeutics.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures







References
-
- Brinbaum LS, Toxicol. Lett 2016, 259, S1.
-
- Suk WA, Heacock ML, Trottier BA, Amolegbe SM, Avakian MD, Carlin DJ, Henry HF, Lopez AR, Skalla LA, Rev. Environ. Health 2020, 35, 85. - PubMed
-
- Heacock ML, Amolegbe SM, Skalla LA, Trottier BA, Carlin DJ, Henry HF, Lopez AR, Duncan CG, Lawler CP, Balshaw DM, Suk WA, Rev. Environ. Health 2020, 35, 111. - PubMed
-
- Baccarelli AA, J. Am. Coll. Cardiol 2019, 74, 1330. - PubMed
-
- WHO Global Strategy on Health, Environment and Climate Change, WHO, Geneva: 2020, pp. 1–36.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

