Lipidomics and Redox Lipidomics Indicate Early Stage Alcohol-Induced Liver Damage
- PMID: 34811964
- PMCID: PMC8870008
- DOI: 10.1002/hep4.1825
Lipidomics and Redox Lipidomics Indicate Early Stage Alcohol-Induced Liver Damage
Abstract
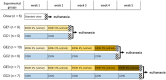
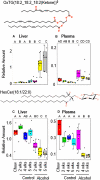
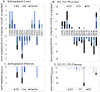
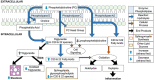
Alcoholic fatty liver disease (AFLD) is characterized by lipid accumulation and inflammation and can progress to cirrhosis and cancer in the liver. AFLD diagnosis currently relies on histological analysis of liver biopsies. Early detection permits interventions that would prevent progression to cirrhosis or later stages of the disease. Herein, we have conducted the first comprehensive time-course study of lipids using novel state-of-the art lipidomics methods in plasma and liver in the early stages of a mouse model of AFLD, i.e., Lieber-DeCarli diet model. In ethanol-treated mice, changes in liver tissue included up-regulation of triglycerides (TGs) and oxidized TGs and down-regulation of phosphatidylcholine, lysophosphatidylcholine, and 20-22-carbon-containing lipid-mediator precursors. An increase in oxidized TGs preceded histological signs of early AFLD, i.e., steatosis, with these changes observed in both the liver and plasma. The major lipid classes dysregulated by ethanol play important roles in hepatic inflammation, steatosis, and oxidative damage. Conclusion: Alcohol consumption alters the liver lipidome before overt histological markers of early AFLD. This introduces the exciting possibility that specific lipids may serve as earlier biomarkers of AFLD than those currently being used.
© 2021 The Authors. Hepatology Communications published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures

References
-
- Ten Hove M, Pater L, Storm G, Weiskirchen S, Weiskirchen R, Lammers T, et al. The hepatic lipidome: from basic science to clinical translation. Adv Drug Deliv Rev 2020;159:180‐197. - PubMed
-
- Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 2006;290:G852‐G858. - PubMed
-
- World Health Organization . Global status report on alcohol and health 2018. https://www.who.int/publications‐detail‐redirect/9789241565639. Published September 27, 2018. Accessed May 2021.
-
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749‐1761. - PubMed
-
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12‐month alcohol use, high‐risk drinking, and DSM‐IV alcohol use disorder in the United States, 2001‐2002 to 2012‐2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74:911‐923. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

