Serum metabolite profiling yields insights into health promoting effect of A. muciniphila in human volunteers with a metabolic syndrome
- PMID: 34812127
- PMCID: PMC8632301
- DOI: 10.1080/19490976.2021.1994270
Serum metabolite profiling yields insights into health promoting effect of A. muciniphila in human volunteers with a metabolic syndrome
Abstract
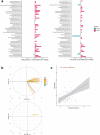
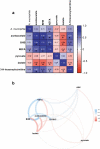
Reduction of A. muciniphila relative abundance in the gut microbiota is a widely accepted signature associated with obesity-related metabolic disorders. Using untargeted metabolomics profiling of fasting plasma, our study aimed at identifying metabolic signatures associated with beneficial properties of alive and pasteurized A. muciniphila when administrated to a cohort of insulin-resistant individuals with metabolic syndrome. Our data highlighted either shared or specific alterations in the metabolome according to the form of A. muciniphila administered with respect to a control group. Common responses encompassed modulation of amino acid metabolism, characterized by reduced levels of arginine and alanine, alongside several intermediates of tyrosine, phenylalanine, tryptophan, and glutathione metabolism. The global increase in levels of acylcarnitines together with specific modulation of acetoacetate also suggested induction of ketogenesis through enhanced β-oxidation. Moreover, our data pinpointed some metabolites of interest considering their emergence as substantial compounds pertaining to health and diseases in the more recent literature.
Keywords: A. muciniphila; acylcarnitines; amino-acids; human; ketone bodies; metabolic syndrome; metabolomic; obesity; prediabetes.
Figures



References
-
- Depommier C, Vitale RM, Iannotti FA, Silvestri C, Flamand N, Druart C, Everard A, Pelicaean R, Maiter D, Thissen JP, et al. Beneficial effects of akkermansia muciniphila are not associated with major changes in the circulating endocannabinoidome but linked to higher mono-palmitoyl-glycerol levels as new PPARα agonists. Cells. 2021;10(1):185. PMID:. doi:10.3390/cells10010185. - DOI - PMC - PubMed
-
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. PMID: 23671105. doi:10.1073/pnas.1219451110. - DOI - PMC - PubMed
-
- Corb Aron RA, Abid A, Vesa CM, Nechifor AC, Behl T, Ghitea TC, Munteanu MA, Fratila O, Andronie-Cioara FL, Toma MM et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of akkermansia muciniphila as a key gut bacterium. Microorganisms. 2021;9. PMID: 33802777. doi:10.3390/microorganisms9030618. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
