Trainee Principal Investigator Could Improve Recruitment in Trauma Trials: Review of Literature and Experience From a Trauma Center
- PMID: 34812304
- PMCID: PMC8604084
- DOI: 10.7759/cureus.18920
Trainee Principal Investigator Could Improve Recruitment in Trauma Trials: Review of Literature and Experience From a Trauma Center
Abstract
Introduction: Recruitment of patients to participate in randomized control trials (RCT) is a challenging task, especially for trauma trials in which the identification and recruitment are time-limited. Multiple strategies have been tried to improve the participation of doctors and the recruitment of patients. The aim was to study the effect of a trainee principal investigator (TPI) on the efficacy of recruitment for a multicenter hip fracture RCT.
Methods: A retrospective study comparing the number of junior doctors participating in the WHiTE 8 COPAL RCT and patients recruited before and after the introduction of formal TPI role at a major trauma center in the UK. Data was collected for nine months "before" (Nov 2018-July 2019) and six months "after" (Sept 2019-Feb 2020) the role of TPI was assigned.
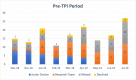
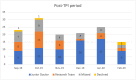
Results: From November 2018 to February 2020, a total of 292 patients were eligible for recruitment into this trial, out of which 196 (67.12 %) were successfully recruited. Excluding the research team, there were seven junior doctors actively recruiting in the "before period" in comparison with 10 in the "after period." Significantly more patients were recruited by junior doctors after a TPI was assigned. Overall, more percentage of eligible patients were recruited into the trial after a TPI was assigned, and this was statistically significant.
Conclusion: The allocation of a formal TPI significantly improved the recruitment of patients in a national RCT. TPI can work alongside the principal investigator and research team to be a valuable link person coordinating and engaging local trainees to take part in trials.
Keywords: junior doctor; randomized control trial; recruitment; trainee principal investigator; trauma trial.
Copyright © 2021, Vas et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Short report: how often do UK primary care trials face recruitment delays? Bower P, Wilson S, Mathers N. Fam Pract. 2007;24:601–603. - PubMed
-
- How evidence based are recruitment strategies to randomized controlled trials in primary care? Experience from seven studies. Foy R, Parry J, Duggan A, et al. Fam Pract. 2003;20:83–92. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
