Major Outer Membrane Protein from Legionella pneumophila Inhibits Phagocytosis but Enhances Chemotaxis of RAW 264.7 Macrophages by Regulating the FOXO1/Coronin-1 Axis
- PMID: 34812410
- PMCID: PMC8605921
- DOI: 10.1155/2021/9409777
Major Outer Membrane Protein from Legionella pneumophila Inhibits Phagocytosis but Enhances Chemotaxis of RAW 264.7 Macrophages by Regulating the FOXO1/Coronin-1 Axis
Abstract
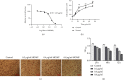
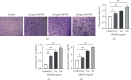
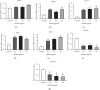
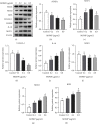
Legionella pneumophila is an intracellular pathogen that can cause Legionnaire's disease by invading alveolar epithelial cells and macrophages. The major outer membrane protein (MOMP) plays an important role in the interaction between bacteria and host cells. However, the role of MOMP in the process of L. pneumophila invasion of macrophages and its working mechanism remain unknown. We aimed to explore the effects of MOMP on phagocytosis and chemotaxis of RAW 264.7 macrophages. The chemotactic activity, toxicity, and phagocytosis of RAW 264.7 cocultured with different concentrations of MOMP were determined by Transwell, CCK-8, and neutral red uptake assays, respectively. Target genes were detected by double-luciferase and pull down assays. qRT-PCR and Western blot were performed to analyze the expression of several important proteins involved in the immune response pathway, including coronin-1, interleukins (IL-10), forkhead transcription factor 1 (FOXO1), nucleotide-binding oligomerization domain protein (NOD) 1, NOD2, and receptor-interacting protein (RIP) 2. After coculturing with MOMP, cytological observation indicated a decrease of phagocytosis and a marked increase of chemotaxis in RAW 264.7 macrophages. The phagocytosis degree of RAW 264.7 macrophage varied with the concentration gradient of MOMP in a time-dependent manner. MOMP could increase the expression levels of MCP-1, IL-10, NOD2, and RIP2 and decrease the expression levels of FOXO1 and coronin-1 in cell culture supernatants. In addition, we found that FOXO1 could promote its transcription by binding to the promoter of coronin-1. The results of the present study suggested that MOMP could inhibit phagocytosis and facilitate chemotaxis of RAW 264.7 macrophage, which might be associated with the FOXO1/coronin-1 axis.
Copyright © 2021 Zehui Yang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
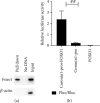
Similar articles
-
[MOMP of Legionella inhibits phagocytosis of RAW264.7 macrophages and enhances their chemotaxis by activating NOD2/RIP2 signaling pathway].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018 Jun;34(6):488-494. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018. PMID: 30236199 Chinese.
-
Exclusion of actin-binding protein p57/coronin-1 from bacteria-containing phagosomes in macrophages infected with Legionella.Biol Pharm Bull. 2008 May;31(5):861-5. doi: 10.1248/bpb.31.861. Biol Pharm Bull. 2008. PMID: 18451508
-
Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone.J Clin Invest. 1984 Sep;74(3):771-82. doi: 10.1172/JCI111493. J Clin Invest. 1984. PMID: 6470140 Free PMC article.
-
[Immunopathogenesis of Legionnaires' disease].Mikrobiyol Bul. 2004 Jul;38(3):295-303. Mikrobiyol Bul. 2004. PMID: 15490851 Review. Turkish.
-
From amoeba to macrophages: exploring the molecular mechanisms of Legionella pneumophila infection in both hosts.Curr Top Microbiol Immunol. 2013;376:1-34. doi: 10.1007/82_2013_351. Curr Top Microbiol Immunol. 2013. PMID: 23949285 Review.
Cited by
-
Role of Legionella pneumophila outer membrane vesicles in host-pathogen interaction.Front Microbiol. 2023 Sep 25;14:1270123. doi: 10.3389/fmicb.2023.1270123. eCollection 2023. Front Microbiol. 2023. PMID: 37817751 Free PMC article. Review.
-
Legionella pneumophila: The Journey from the Environment to the Blood.J Clin Med. 2022 Oct 18;11(20):6126. doi: 10.3390/jcm11206126. J Clin Med. 2022. PMID: 36294446 Free PMC article. Review.
References
-
- Arnold I. C., Paul W. D., Genhong C. Rip2: a key molecule that regulates both innate and acquired immunity. Current Medicinal Chemistry - Anti-Inflammatory & Anti-Allergy Agents . 2005;4(1):35–42. doi: 10.2174/1568014053005264. - DOI
-
- Bellinger-Kawahara C., Horwitz M. A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. The Journal of Experimental Medicine . 1990;172(4):1201–1210. doi: 10.1084/jem.172.4.1201. - DOI - PMC - PubMed
-
- Krinos C., High A. S., Rodgers F. G. Role of the 25 kDa major outer membrane protein of Legionella pneumophila in attachment to U-937 cells and its potential as a virulence factor for chick embryos. Journal of Applied Microbiology . 1999;86(2):237–244. doi: 10.1046/j.1365-2672.1999.00667.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

