Deficiency of the paternally inherited gene Magel2 alters the development of separation-induced vocalization and maternal behavior in mice
- PMID: 34812568
- PMCID: PMC9744533
- DOI: 10.1111/gbb.12776
Deficiency of the paternally inherited gene Magel2 alters the development of separation-induced vocalization and maternal behavior in mice
Abstract
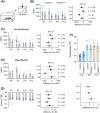
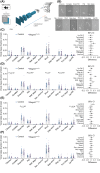
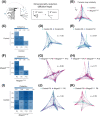
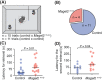
The behavior of offspring results from the combined expression of maternal and paternal genes. Genomic imprinting silences some genes in a parent-of-origin specific manner, a process that, among all animals, occurs only in mammals. How genomic imprinting affects the behavior of mammalian offspring, however, remains poorly understood. Here, we studied how the loss of the paternally inherited gene Magel2 in mouse pups affects the emission of separation-induced ultrasonic vocalizations (USV). Using quantitative analysis of more than 1000 USVs, we characterized the rate of vocalizations as well as their spectral features from postnatal days 6-12 (P6-P12), a critical phase of mouse development that covers the peak of vocal behavior in pups. Our analyses show that Magel2 deficient offspring emit separation-induced vocalizations at lower rates and with altered spectral features mainly at P8. We also show that dams display altered behavior towards their own Magel2 deficient offspring at this age. In a test to compare the retrieval of two pups, dams retrieve wildtype control pups first and faster than Magel2 deficient offspring. These results suggest that the loss of Magel2 impairs the expression of separation-induced vocalization in pups as well as maternal behavior at a specific age of postnatal development, both of which support the pups' growth and development.
Keywords: Prader Willi syndrome; autism spectrum disorders; behavior development; genomic imprinting; offspring-parent conflict.
© 2021 The Authors. Genes, Brain and Behavior published by International Behavioural and Neural Genetics Society and John Wiley & Sons Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

