Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion
- PMID: 34812647
- PMCID: PMC9835999
- DOI: 10.1126/sciimmunol.abl9929
Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion
Abstract
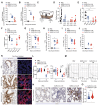
The development of a tractable small animal model faithfully reproducing human coronavirus disease 2019 pathogenesis would arguably meet a pressing need in biomedical research. Thus far, most investigators have used transgenic mice expressing the human ACE2 in epithelial cells (K18-hACE2 transgenic mice) that are intranasally instilled with a liquid severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) suspension under deep anesthesia. Unfortunately, this experimental approach results in disproportionate high central nervous system infection leading to fatal encephalitis, which is rarely observed in humans and severely limits this model’s usefulness. Here, we describe the use of an inhalation tower system that allows exposure of unanesthetized mice to aerosolized virus under controlled conditions. Aerosol exposure of K18-hACE2 transgenic mice to SARS-CoV-2 resulted in robust viral replication in the respiratory tract, anosmia, and airway obstruction but did not lead to fatal viral neuroinvasion. When compared with intranasal inoculation, aerosol infection resulted in a more pronounced lung pathology including increased immune infiltration, fibrin deposition, and a transcriptional signature comparable to that observed in SARS-CoV-2–infected patients. This model may prove useful for studies of viral transmission, disease pathogenesis (including long-term consequences of SARS-CoV-2 infection), and therapeutic interventions.
Figures

References
-
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E. C., Zhang Y.-Z., A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020). 10.1038/s41586-020-2008-3 - DOI - PMC - PubMed
-
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). 10.1038/s41586-020-2012-7 - DOI - PMC - PubMed
-
- Qiu C., Cui C., Hautefort C., Haehner A., Zhao J., Yao Q., Zeng H., Nisenbaum E. J., Liu L., Zhao Y., Zhang D., Levine C. G., Cejas I., Dai Q., Zeng M., Herman P., Jourdaine C., de With K., Draf J., Chen B., Jayaweera D. T., Denneny J. C. 3rd, Casiano R., Yu H., Eshraghi A. A., Hummel T., Liu X., Shu Y., Lu H., Olfactory and Gustatory Dysfunction as an Early Identifier of COVID-19 in Adults and Children: An International Multicenter Study. Otolaryngol. Head Neck Surg. 163, 714–721 (2020). 10.1177/0194599820934376 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

