Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial
- PMID: 34812653
- PMCID: PMC9017870
- DOI: 10.1126/science.abm3425
Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial
Abstract
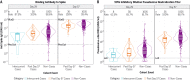
In the coronavirus efficacy (COVE) phase 3 clinical trial, vaccine recipients were assessed for neutralizing and binding antibodies as correlates of risk for COVID-19 disease and as correlates of protection. These immune markers were measured at the time of second vaccination and 4 weeks later, with values reported in standardized World Health Organization international units. All markers were inversely associated with COVID-19 risk and directly associated with vaccine efficacy. Vaccine recipients with postvaccination 50% neutralization titers 10, 100, and 1000 had estimated vaccine efficacies of 78% (95% confidence interval, 54 to 89%), 91% (87 to 94%), and 96% (94 to 98%), respectively. These results help define immune marker correlates of protection and may guide approval decisions for messenger RNA (mRNA) COVID-19 vaccines and other COVID-19 vaccines.
Figures



Update of
-
Immune Correlates Analysis of the mRNA-1273 COVID-19 Vaccine Efficacy Trial.medRxiv [Preprint]. 2021 Aug 15:2021.08.09.21261290. doi: 10.1101/2021.08.09.21261290. medRxiv. 2021. Update in: Science. 2022 Jan 07;375(6576):43-50. doi: 10.1126/science.abm3425. PMID: 34401888 Free PMC article. Updated. Preprint.
Comment in
-
Using correlates to accelerate vaccinology.Science. 2022 Jan 7;375(6576):22-23. doi: 10.1126/science.abn0007. Epub 2022 Jan 6. Science. 2022. PMID: 34990231
References
-
- World Health Organization, “Coronavirus Disease (COVID-19): COVID-19 vaccine EUL issued” (2021); https://extranet.who.int/pqweb/vaccines/covid-19-vaccines.
-
- US Food and Drug Administration (FDA), “COVID-19 Vaccines: COVID-19 Vaccines Authorized for Emergency Use” (2021); www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019... [last updated 11 March 2021].
-
- US Food and Drug Administration, “FDA Approves First COVID-19 Vaccine: Approval Signifies Key Achievement for Public Health” (FDA news release, 23 August 2021); www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-....
-
- C. Weller, “Four reasons why we need multiple vaccines for Covid-19” (Wellcome Opinion, 6 June 2021); https://wellcome.org/news/four-reasons-why-we-need-multiple-vaccines-cov....
-
- Wouters O. J., Shadlen K. C., Salcher-Konrad M., Pollard A. J., Larson H. J., Teerawattananon Y., Jit M., Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 397, 1023–1034 (2021). 10.1016/S0140-6736(21)00306-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

