Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial
- PMID: 34812863
- PMCID: PMC8611484
- DOI: 10.1001/jama.2021.19415
Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial
Abstract
Importance: Most clinical guidelines do not recommend platelet-rich plasma (PRP) for knee osteoarthritis (OA) because of lack of high-quality evidence on efficacy for symptoms and joint structure, but the guidelines emphasize the need for rigorous studies. Despite this, use of PRP in knee OA is increasing.
Objective: To evaluate the effects of intra-articular PRP injections on symptoms and joint structure in patients with symptomatic mild to moderate radiographic medial knee OA.
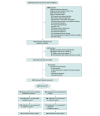
Design, setting, and participants: This randomized, 2-group, placebo-controlled, participant-, injector-, and assessor-blinded clinical trial enrolled community-based participants (n = 288) aged 50 years or older with symptomatic medial knee OA (Kellgren and Lawrence grade 2 or 3) in Sydney and Melbourne, Australia, from August 24, 2017, to July 5, 2019. The 12-month follow-up was completed on July 22, 2020.
Interventions: Interventions involved 3 intra-articular injections at weekly intervals of either leukocyte-poor PRP using a commercially available product (n = 144 participants) or saline placebo (n = 144 participants).
Main outcomes and measures: The 2 primary outcomes were 12-month change in overall average knee pain scores (11-point scale; range, 0-10, with higher scores indicating worse pain; minimum clinically important difference of 1.8) and percentage change in medial tibial cartilage volume as assessed by magnetic resonance imaging (MRI). Thirty-one secondary outcomes (25 symptom related and 6 MRI assessed; minimum clinically important difference not known) evaluated pain, function, quality of life, global change, and joint structures at 2-month and/or 12-month follow-up.
Results: Among 288 patients who were randomized (mean age, 61.9 [SD, 6.5] years; 169 [59%] women), 269 (93%) completed the trial. In both groups, 140 participants (97%) received all 3 injections. After 12 months, treatment with PRP vs placebo injection resulted in a mean change in knee pain scores of -2.1 vs -1.8 points, respectively (difference, -0.4 [95% CI, -0.9 to 0.2] points; P = .17). The mean change in medial tibial cartilage volume was -1.4% vs -1.2%, respectively (difference, -0.2% [95% CI, -1.9% to 1.5%]; P = .81). Of 31 prespecified secondary outcomes, 29 showed no significant between-group differences.
Conclusions and relevance: Among patients with symptomatic mild to moderate radiographic knee OA, intra-articular injection of PRP, compared with injection of saline placebo, did not result in a significant difference in symptoms or joint structure at 12 months. These findings do not support use of PRP for the management of knee OA.
Trial registration: Australian New Zealand Clinical Trials Registry Identifier: ACTRN12617000853347.
Conflict of interest statement
Figures

Comment in
-
Platelet-Rich Plasma for Osteoarthritis and Achilles Tendinitis.JAMA. 2021 Nov 23;326(20):2012-2014. doi: 10.1001/jama.2021.19540. JAMA. 2021. PMID: 34812886 No abstract available.
-
Use of platelet-rich plasma for knee OA not supported by RCT results.Nat Rev Rheumatol. 2022 Feb;18(2):61. doi: 10.1038/s41584-021-00743-7. Nat Rev Rheumatol. 2022. PMID: 34983976 No abstract available.
-
Intra-articular Platelet-Rich Plasma vs Placebo Injection and Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis.JAMA. 2022 Mar 22;327(12):1186-1187. doi: 10.1001/jama.2022.1312. JAMA. 2022. PMID: 35315893 No abstract available.
-
Intra-articular Platelet-Rich Plasma vs Placebo Injection and Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis.JAMA. 2022 Mar 22;327(12):1186. doi: 10.1001/jama.2022.1309. JAMA. 2022. PMID: 35315894 No abstract available.
-
Intra-articular Platelet-Rich Plasma vs Placebo Injection and Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis.JAMA. 2022 Mar 22;327(12):1184-1185. doi: 10.1001/jama.2022.1306. JAMA. 2022. PMID: 35315895 No abstract available.
-
Intra-articular Platelet-Rich Plasma vs Placebo Injection and Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis.JAMA. 2022 Mar 22;327(12):1185-1186. doi: 10.1001/jama.2022.1303. JAMA. 2022. PMID: 35315896 No abstract available.
-
Comment on "Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients with Knee Osteoarthritis: The Restore Randomized Clinical Trial".Pain Physician. 2022 Jul;25(4):E698. Pain Physician. 2022. PMID: 35793195 No abstract available.
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

