Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis
- PMID: 34813277
- PMCID: PMC8796287
- DOI: 10.1021/acs.chemrev.1c00374
Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis
Abstract
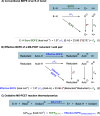
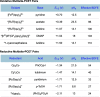
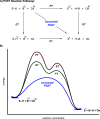
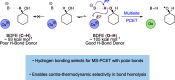
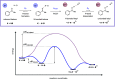
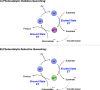
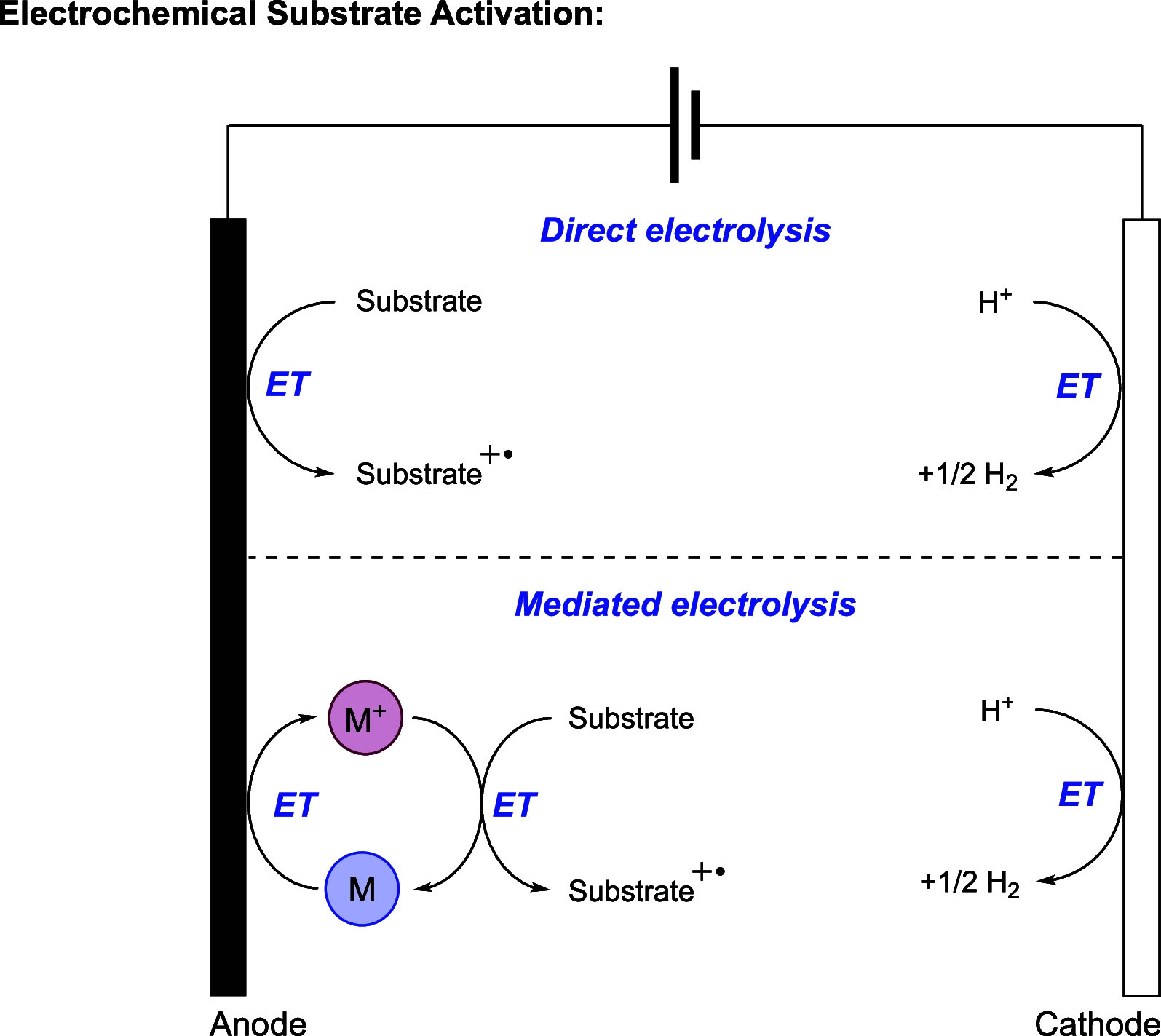
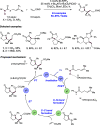
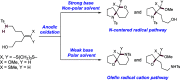
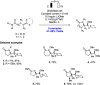
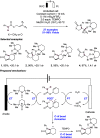
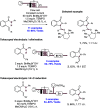
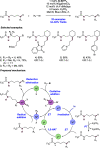
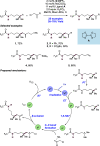
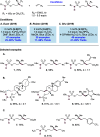
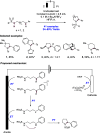
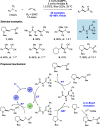
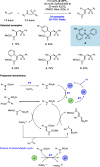
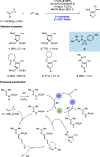
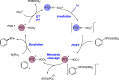
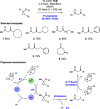
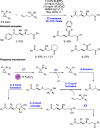
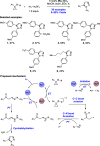
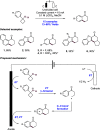
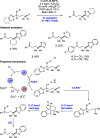
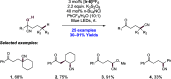
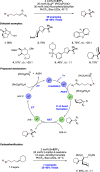
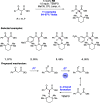
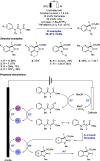
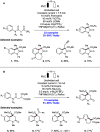
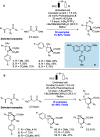
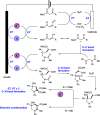
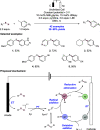
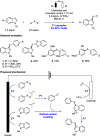
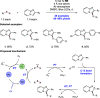
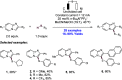
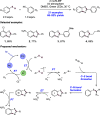
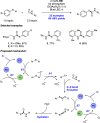
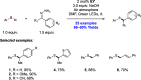
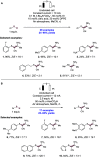
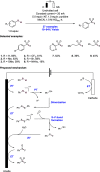
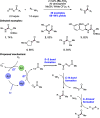
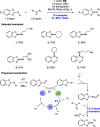
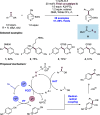
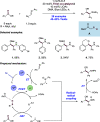
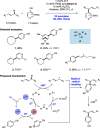
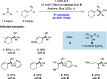
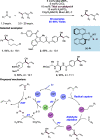
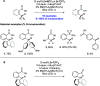
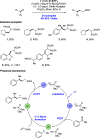
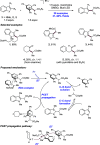
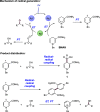
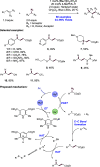
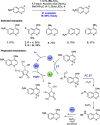
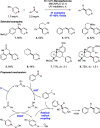
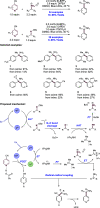
We present here a review of the photochemical and electrochemical applications of multi-site proton-coupled electron transfer (MS-PCET) in organic synthesis. MS-PCETs are redox mechanisms in which both an electron and a proton are exchanged together, often in a concerted elementary step. As such, MS-PCET can function as a non-classical mechanism for homolytic bond activation, providing opportunities to generate synthetically useful free radical intermediates directly from a wide variety of common organic functional groups. We present an introduction to MS-PCET and a practitioner's guide to reaction design, with an emphasis on the unique energetic and selectivity features that are characteristic of this reaction class. We then present chapters on oxidative N-H, O-H, S-H, and C-H bond homolysis methods, for the generation of the corresponding neutral radical species. Then, chapters for reductive PCET activations involving carbonyl, imine, other X═Y π-systems, and heteroarenes, where neutral ketyl, α-amino, and heteroarene-derived radicals can be generated. Finally, we present chapters on the applications of MS-PCET in asymmetric catalysis and in materials and device applications. Within each chapter, we subdivide by the functional group undergoing homolysis, and thereafter by the type of transformation being promoted. Methods published prior to the end of December 2020 are presented.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


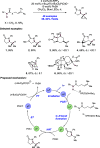




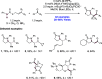
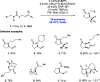
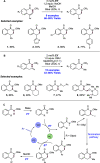





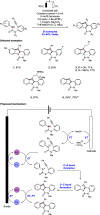
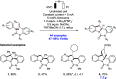


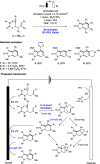
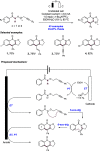

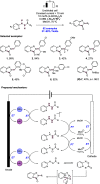
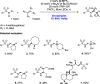

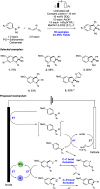

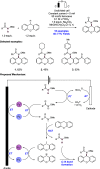

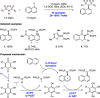
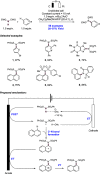
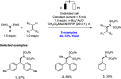

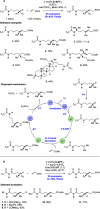


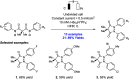
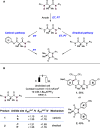
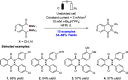
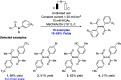
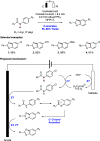
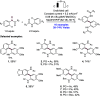
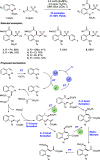
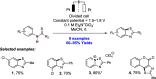


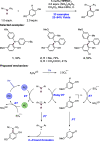
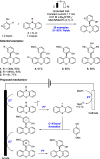
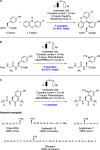
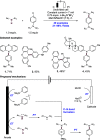





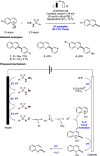


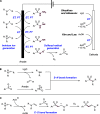
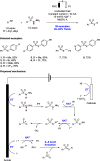
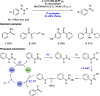
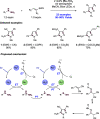
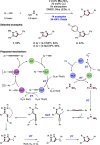
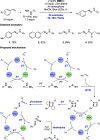


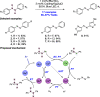

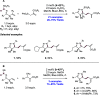
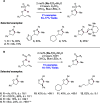
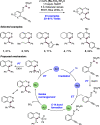
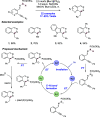



















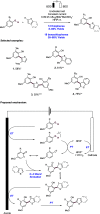
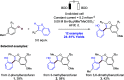
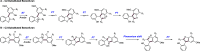
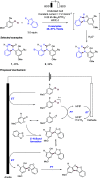





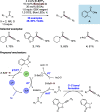
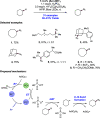
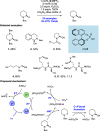
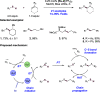




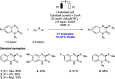

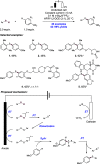
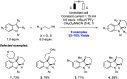
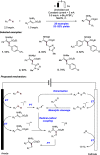













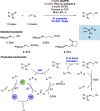
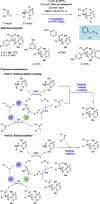
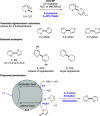

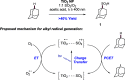




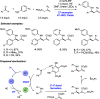

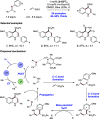
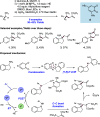
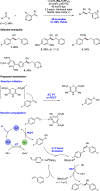












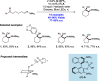


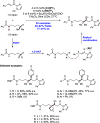
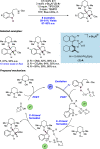
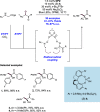





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

