Positive and negative selection shape the human naive B cell repertoire
- PMID: 34813502
- PMCID: PMC8759783
- DOI: 10.1172/JCI150985
Positive and negative selection shape the human naive B cell repertoire
Abstract
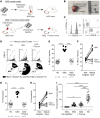
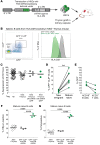
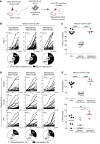
Although negative selection of developing B cells in the periphery is well described, yet poorly understood, evidence of naive B cell positive selection remains elusive. Using 2 humanized mouse models, we demonstrate that there was strong skewing of the expressed immunoglobulin repertoire upon transit into the peripheral naive B cell pool. This positive selection of expanded naive B cells in humanized mice resembled that observed in healthy human donors and was independent of autologous thymic tissue. In contrast, negative selection of autoreactive B cells required thymus-derived Tregs and MHC class II-restricted self-antigen presentation by B cells. Indeed, both defective MHC class II expression on B cells of patients with rare bare lymphocyte syndrome and prevention of self-antigen presentation via HLA-DM inhibition in humanized mice resulted in the production of autoreactive naive B cells. These latter observations suggest that Tregs repressed autoreactive naive B cells continuously produced by the bone marrow. Thus, a model emerged, in which both positive and negative selection shaped the human naive B cell repertoire and that each process was mediated by fundamentally different molecular and cellular mechanisms.
Keywords: Autoimmunity; Immunology; Tolerance.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

