p300 or CBP is required for insulin-stimulated glucose uptake in skeletal muscle and adipocytes
- PMID: 34813504
- PMCID: PMC8765050
- DOI: 10.1172/jci.insight.141344
p300 or CBP is required for insulin-stimulated glucose uptake in skeletal muscle and adipocytes
Abstract
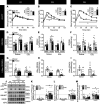
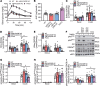
While current thinking posits that insulin signaling to glucose transporter 4 (GLUT4) exocytic translocation and glucose uptake in skeletal muscle and adipocytes is controlled by phosphorylation-based signaling, many proteins in this pathway are acetylated on lysine residues. However, the importance of acetylation and lysine acetyltransferases to insulin-stimulated glucose uptake is incompletely defined. Here, we demonstrate that combined loss of the acetyltransferases E1A binding protein p300 (p300) and cAMP response element binding protein binding protein (CBP) in mouse skeletal muscle caused a complete loss of insulin-stimulated glucose uptake. Similarly, brief (i.e., 1 hour) pharmacological inhibition of p300/CBP acetyltransferase activity recapitulated this phenotype in human and rodent myotubes, 3T3-L1 adipocytes, and mouse muscle. Mechanistically, these effects were due to p300/CBP-mediated regulation of GLUT4 exocytic translocation and occurred downstream of Akt signaling. Taken together, we highlight a fundamental role for acetylation and p300/CBP in the direct regulation of insulin-stimulated glucose transport in skeletal muscle and adipocytes.
Keywords: Endocrinology; Glucose metabolism; Insulin signaling; Muscle Biology; Skeletal muscle.
Conflict of interest statement
Figures



Similar articles
-
Insights into posttranslational regulation of skeletal muscle contractile function by the acetyltransferases, p300 and CBP.J Appl Physiol (1985). 2024 Jun 1;136(6):1559-1567. doi: 10.1152/japplphysiol.00156.2024. Epub 2024 May 9. J Appl Physiol (1985). 2024. PMID: 38722753 Free PMC article.
-
Germline or inducible knockout of p300 or CBP in skeletal muscle does not alter insulin sensitivity.Am J Physiol Endocrinol Metab. 2019 Jun 1;316(6):E1024-E1035. doi: 10.1152/ajpendo.00497.2018. Epub 2019 Mar 19. Am J Physiol Endocrinol Metab. 2019. PMID: 30888860 Free PMC article.
-
p300 and cAMP response element-binding protein-binding protein in skeletal muscle homeostasis, contractile function, and survival.J Cachexia Sarcopenia Muscle. 2020 Apr;11(2):464-477. doi: 10.1002/jcsm.12522. Epub 2020 Jan 3. J Cachexia Sarcopenia Muscle. 2020. PMID: 31898871 Free PMC article.
-
Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport.Appl Physiol Nutr Metab. 2007 Jun;32(3):557-66. doi: 10.1139/H07-026. Appl Physiol Nutr Metab. 2007. PMID: 17510697 Review.
-
Adrenoceptors promote glucose uptake into adipocytes and muscle by an insulin-independent signaling pathway involving mechanistic target of rapamycin complex 2.Pharmacol Res. 2017 Feb;116:87-92. doi: 10.1016/j.phrs.2016.12.022. Epub 2016 Dec 23. Pharmacol Res. 2017. PMID: 28025104 Review.
Cited by
-
The epigenetic regulatory effect of histone acetylation and deacetylation on skeletal muscle metabolism-a review.Front Physiol. 2023 Dec 8;14:1267456. doi: 10.3389/fphys.2023.1267456. eCollection 2023. Front Physiol. 2023. PMID: 38148899 Free PMC article. Review.
-
Maintenance of thermogenic adipose tissues despite loss of the H3K27 acetyltransferases p300 or CBP.Am J Physiol Endocrinol Metab. 2024 Oct 1;327(4):E459-E468. doi: 10.1152/ajpendo.00120.2024. Epub 2024 Aug 14. Am J Physiol Endocrinol Metab. 2024. PMID: 39140972 Free PMC article.
-
Insights into posttranslational regulation of skeletal muscle contractile function by the acetyltransferases, p300 and CBP.J Appl Physiol (1985). 2024 Jun 1;136(6):1559-1567. doi: 10.1152/japplphysiol.00156.2024. Epub 2024 May 9. J Appl Physiol (1985). 2024. PMID: 38722753 Free PMC article.
-
p300 nucleocytoplasmic shuttling underlies mTORC1 hyperactivation in Hutchinson-Gilford progeria syndrome.Nat Cell Biol. 2024 Feb;26(2):235-249. doi: 10.1038/s41556-023-01338-y. Epub 2024 Jan 24. Nat Cell Biol. 2024. PMID: 38267537 Free PMC article.
-
Intrinsic Skeletal Muscle Function and Contraction-Stimulated Glucose Uptake Do Not Vary by Time-of-Day in Mice.Function (Oxf). 2024 Nov 20;5(6):zqae035. doi: 10.1093/function/zqae035. Function (Oxf). 2024. PMID: 39134511 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

