Modeling SARS-CoV-2 propagation using rat coronavirus-associated shedding and transmission
- PMID: 34813610
- PMCID: PMC8610237
- DOI: 10.1371/journal.pone.0260038
Modeling SARS-CoV-2 propagation using rat coronavirus-associated shedding and transmission
Abstract
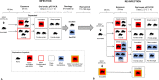
At present, global immunity to SARS-CoV-2 resides within a heterogeneous combination of susceptible, naturally infected and vaccinated individuals. The extent to which viral shedding and transmission occurs on re-exposure to SARS-CoV-2 is an important determinant of the rate at which COVID-19 achieves endemic stability. We used Sialodacryoadenitis Virus (SDAV) in rats to model the extent to which immune protection afforded by prior natural infection via high risk (inoculation; direct contact) or low risk (fomite) exposure, or by vaccination, influenced viral shedding and transmission on re-exposure. On initial infection, we confirmed that amount, duration and consistency of viral shedding, and seroconversion rates were correlated with exposure risk. Animals were reinfected after 3.7-5.5 months using the same exposure paradigm. 59% of seropositive animals shed virus, although at lower amounts. Previously exposed seropositive reinfected animals were able to transmit virus to 25% of naive recipient rats after 24-hour exposure by direct contact. Rats vaccinated intranasally with a related virus (Parker's Rat Coronavirus) were able to transmit SDAV to only 4.7% of naive animals after a 7-day direct contact exposure, despite comparable viral shedding. Cycle threshold values associated with transmission in both groups ranged from 29-36 cycles. Observed shedding was not a prerequisite for transmission. Results indicate that low-level shedding in both naturally infected and vaccinated seropositive animals can propagate infection in susceptible individuals. Extrapolated to COVID-19, our results suggest that continued propagation of SARS-CoV-2 by seropositive previously infected or vaccinated individuals is possible.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

