Pulmonary surfactant as a versatile biomaterial to fight COVID-19
- PMID: 34813878
- PMCID: PMC8605818
- DOI: 10.1016/j.jconrel.2021.11.023
Pulmonary surfactant as a versatile biomaterial to fight COVID-19
Abstract
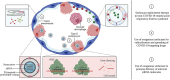
The COVID-19 pandemic has wielded an enormous pressure on global health care systems, economics and politics. Ongoing vaccination campaigns effectively attenuate viral spreading, leading to a reduction of infected individuals, hospitalizations and mortality. Nevertheless, the development of safe and effective vaccines as well as their global deployment is time-consuming and challenging. In addition, such preventive measures have no effect on already infected individuals and can show reduced efficacy against SARS-CoV-2 variants that escape vaccine-induced host immune responses. Therefore, it is crucial to continue the development of specific COVID-19 targeting therapeutics, including small molecular drugs, antibodies and nucleic acids. However, despite clear advantages of local drug delivery to the lung, inhalation therapy of such antivirals remains difficult. This review aims to highlight the potential of pulmonary surfactant (PS) in the treatment of COVID-19. Since SARS-CoV-2 infection can progress to COVID-19-related acute respiratory distress syndrome (CARDS), which is associated with PS deficiency and inflammation, replacement therapy with exogenous surfactant can be considered to counter lung dysfunction. In addition, due to its surface-active properties and membrane-interacting potential, PS can be repurposed to enhance drug spreading along the respiratory epithelium and to promote intracellular drug delivery. By merging these beneficial features, PS can be regarded as a versatile biomaterial to combat respiratory infections, in particular COVID-19.
Keywords: Antiviral drugs; Coronavirus disease-19; Inhalation therapy; Lung delivery; Nanomedicine; Pulmonary surfactant; Severe acute respiratory syndrome coronavirus-2; Small-interfering RNA.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures







References
-
- World Health Organization . WHO COVID-19 Dashboard [Internet] WHO; 2021. https://covid19.who.int/
-
- Veldhuizen R.A.W., Zuo Y.Y., Petersen N.O., Lewis J.F., Possmayer F. The COVID-19 pandemic: a target for surfactant therapy? Expert Rev. Respir. Med. 2021;15:597–608. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

