A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity
- PMID: 34814158
- PMCID: PMC8791831
- DOI: 10.1038/s41586-021-04232-5
A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity
Abstract
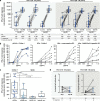
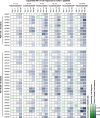
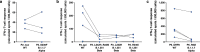
T cell immunity is central for the control of viral infections. CoVac-1 is a peptide-based vaccine candidate, composed of SARS-CoV-2 T cell epitopes derived from various viral proteins1,2, combined with the Toll-like receptor 1/2 agonist XS15 emulsified in Montanide ISA51 VG, aiming to induce profound SARS-CoV-2 T cell immunity to combat COVID-19. Here we conducted a phase I open-label trial, recruiting 36 participants aged 18-80 years, who received a single subcutaneous CoVac-1 vaccination. The primary end point was safety analysed until day 56. Immunogenicity in terms of CoVac-1-induced T cell response was analysed as the main secondary end point until day 28 and in the follow-up until month 3. No serious adverse events and no grade 4 adverse events were observed. Expected local granuloma formation was observed in all study participants, whereas systemic reactogenicity was absent or mild. SARS-CoV-2-specific T cell responses targeting multiple vaccine peptides were induced in all study participants, mediated by multifunctional T helper 1 CD4+ and CD8+ T cells. CoVac-1-induced IFNγ T cell responses persisted in the follow-up analyses and surpassed those detected after SARS-CoV-2 infection as well as after vaccination with approved vaccines. Furthermore, vaccine-induced T cell responses were unaffected by current SARS-CoV-2 variants of concern. Together, CoVac-1 showed a favourable safety profile and induced broad, potent and variant of concern-independent T cell responses, supporting the presently ongoing evaluation in a phase II trial for patients with B cell or antibody deficiency.
© 2022. The Author(s).
Conflict of interest statement
The University Hospital Tübingen is in the process of applying for a patent application (EP 20 169 047.6) covering the SARS-CoV-2 T cell epitopes included in CoVac-1 that lists A.N., T.B., H.-G.R. and J.S.W as inventors. EMC Microcollections GmbH is in the process of applying for a patent application (DE102016005550.2) covering the adjuvant XS15 included in CoVac-1 that lists H.-G.R. as an inventor. The other authors declare no competing interests.
Figures






References
-
- Nelde A, et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021;22:74–85. - PubMed
-
- WHO. Weekly epidemiological update on COVID-19. WHOhttps://www.who.int/docs/default-source/coronaviruse/situation-reports/2... (2021).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

