Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial
- PMID: 34815219
- PMCID: PMC8862056
- DOI: 10.1136/annrheumdis-2021-221048
Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial
Abstract
Objectives: Risankizumab is an interleukin-23 inhibitor under study for the treatment of patients with psoriatic arthritis (PsA). The phase 3 KEEPsAKE 2 trial investigated the efficacy and safety of risankizumab versus placebo in patients with active PsA who had previous inadequate response or intolerance to ≤2 biological therapies (Bio-IR) and/or ≥1 conventional synthetic disease-modifying antirheumatic drug (csDMARD-IR). Results through week 24 are reported here.
Methods: Adults with PsA who were Bio-IR and/or csDMARD-IR were randomised to receive subcutaneously administered risankizumab 150 mg or placebo at weeks 0, 4 and 16 during a 24-week, double-blind treatment period. The primary endpoint was the proportion of patients who achieved ≥20% improvement in American College of Rheumatology score (ACR20) at week 24. Secondary endpoints assessed key domains of PsA and patient-reported outcomes.
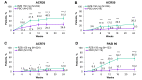
Results: A total of 444 patients (median age 53 years, range 23-84 years) were randomised to risankizumab (n=224) or placebo (n=220); 206 patients (46.5%) were Bio-IR. At week 24, a significantly greater proportion of patients receiving risankizumab achieved the primary endpoint of ACR20 (51.3% vs 26.5%, p<0.001) and all secondary endpoints (p<0.05) compared with placebo. Serious adverse events were reported for 4.0% and 5.5% of risankizumab-treated and placebo-treated patients, respectively; serious infections were reported for 0.9% and 2.3%, respectively.
Conclusion: Treatment with risankizumab resulted in significant improvements versus placebo in key disease outcomes and was well tolerated in patients with PsA who were Bio-IR and/or csDMARD-IR.
Trial registration number: NCT03671148.
Keywords: arthritis; biological therapy; psoriatic.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AO received speaker or consulting fees and/or research grants from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi and UCB. FEVdB received speaker and/or consulting fees from AbbVie, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer and UCB. KP received honoraria or fees for serving on advisory boards, as a speaker and as a consultant. He has received grants for services as principal investigator from AbbVie, Amgen, Astellas, Bausch Health (Valeant), Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, EMD Serono, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda and UCB. CA received research support and honoraria or fees for serving on advisory boards or as a speaker from AbbVie, Amgen, Genentech, Janssen, Lilly, Pfizer and Roche. RB received grants or research support from AbbVie, Merck and Roche. He has received consultation fees or honoraria for serving as a speaker for AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Merck, Pfizer and Roche. JA received honoraria as a consultant, speaker or expert witness. He has received research support from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Galapagos/Gilead, Genentech, GlaxoSmithKline, Lilly, Mallinckrodt, Nektar Therapeutics, Nichi-Iko, Novartis, Pfizer, Regeneron, Roche, Sanofi, Selecta Biosciences and UCB. WL, ZW, AMS, AE and LB are full-time employees of AbbVie, and may hold AbbVie stock or stock options. AMS is listed as an inventor on some AbbVie patents. GA was a full-time employee of AbbVie at the time of this study and may hold AbbVie stock or stock options. AK is a shareholder of Amgen, Gilead, GlaxoSmithKline, Novartis, Pfizer and Sanofi. He received consulting fees from AbbVie, Boehringer Ingleheim, Flexion, Gilead, Janssen, Pfizer, Regeneron, Sanofi and SUN Pharma, and has received speaker’s fees or honoraria from AbbVie, Celgene, Flexion, Genzyme, GlaxoSmithKline, Lilly, Merck, Novartis, Pfizer, Sanofi and UCB.
Figures

Comment in
-
Risankizumab improves PsA.Nat Rev Rheumatol. 2022 Mar;18(3):127. doi: 10.1038/s41584-022-00751-1. Nat Rev Rheumatol. 2022. PMID: 35082372 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

