Evaluation of the safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in HIV-infected pregnant women: protocol of a multicentre, two-arm, randomised, placebo-controlled, superiority clinical trial (MAMAH project)
- PMID: 34815285
- PMCID: PMC8611429
- DOI: 10.1136/bmjopen-2021-053197
Evaluation of the safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in HIV-infected pregnant women: protocol of a multicentre, two-arm, randomised, placebo-controlled, superiority clinical trial (MAMAH project)
Abstract
Introduction: Malaria infection during pregnancy is an important driver of maternal and neonatal health especially among HIV-infected women. Intermittent preventive treatment in pregnancy (IPTp) with sulphadoxine-pyrimethamine is recommended for malaria prevention in HIV-uninfected women, but it is contraindicated in those HIV-infected on cotrimoxazole prophylaxis (CTXp) due to potential adverse effects. Dihydroartemisinin-piperaquine (DHA-PPQ) has been shown to improve antimalarial protection, constituting a promising IPTp candidate. This trial's objective is to determine if monthly 3-day IPTp courses of DHA-PPQ added to daily CTXp are safe and superior to CTXp alone in decreasing the proportion of peripheral malaria parasitaemia at the end of pregnancy.
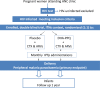
Methods and analysis: This is a multicentre, two-arm, placebo-controlled, individually randomised trial in HIV-infected pregnant women receiving CTXp and antiretroviral treatment. A total of 664 women will be enrolled at the first antenatal care clinic visit in sites from Gabon and Mozambique. Participants will receive an insecticide-treated net, and they will be administered monthly IPTp with DHA-PPQ or placebo (1:1 ratio) as directly observed therapy from the second trimester of pregnancy. Primary study outcome is the prevalence of maternal parasitaemia at delivery. Secondary outcomes include prevalence of malaria-related maternal and infant outcomes and proportion of adverse perinatal outcomes. Participants will be followed until 6 weeks after the end of pregnancy and their infants until 1 year of age to also evaluate the impact of DHA-PPQ on mother-to-child transmission of HIV. The analysis will be done in the intention to treat and according to protocol cohorts, adjusted by gravidity, country, seasonality and other variables associated with malaria.
Ethics and dissemination: The protocol was reviewed and approved by the institutional and national ethics committees of Gabon and Mozambique and the Hospital Clinic of Barcelona. Project results will be presented to all stakeholders and published in open-access journals.
Trial registration number: NCT03671109.
Keywords: HIV & AIDS; clinical trials; epidemiology; maternal medicine; tropical medicine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- WHO . World malaria report 2020. WHO, 2020. Available: https://www.who.int/publications/i/item/9789240015791 [Accessed March 2021].
-
- WWARN Artemisinin based Combination Therapy (ACT) Africa Baseline Study Group, Dahal P, d'Alessandro U, et al. . Clinical determinants of early parasitological response to acts in African patients with uncomplicated falciparum malaria: a literature review and meta-analysis of individual patient data. BMC Med 2015;13:212. 10.1186/s12916-015-0445-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical