Correlated diffusion in lipid bilayers
- PMID: 34815347
- PMCID: PMC8640750
- DOI: 10.1073/pnas.2113202118
Correlated diffusion in lipid bilayers
Abstract
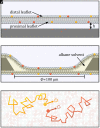
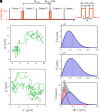
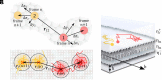
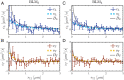
Lipid membranes are complex quasi-two-dimensional fluids, whose importance in biology and unique physical/materials properties have made them a major target for biophysical research. Recent single-molecule tracking experiments in membranes have caused some controversy, calling the venerable Saffman-Delbrück model into question and suggesting that, perhaps, current understanding of membrane hydrodynamics is imperfect. However, single-molecule tracking is not well suited to resolving the details of hydrodynamic flows; observations involving correlations between multiple molecules are superior for this purpose. Here dual-color molecular tracking with submillisecond time resolution and submicron spatial resolution is employed to reveal correlations in the Brownian motion of pairs of fluorescently labeled lipids in membranes. These correlations extend hundreds of nanometers in freely floating bilayers (black lipid membranes) but are severely suppressed in supported lipid bilayers. The measurements are consistent with hydrodynamic predictions based on an extended Saffman-Delbrück theory that explicitly accounts for the two-leaflet bilayer structure of lipid membranes.
Keywords: Saffman–Delbrück model; membrane hydrodynamics; single-molecule tracking.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Gennis R. B., Biomembranes: Molecular Structure and Function (Springer-Verlag, 1989).
-
- Lodish H., et al. , Molecular Cell Biology (Scientific American Books, New York, 1995).
-
- Lipowsky R., Sackmann E., Structure and Dynamics of Membranes (Elsevier Science, 1995).
-
- Nelson D., Piran T., Weinberg S., Statistical Mechanics of Membranes and Surfaces (World Scientific, 2004).
-
- Saffman P. G., Brownian motion in thin sheets of viscous fluid. J. Fluid Mech. 73, 593–602 (1976).
Publication types
LinkOut - more resources
Full Text Sources

