Reversible and spatiotemporal control of colloidal structure formation
- PMID: 34815410
- PMCID: PMC8611085
- DOI: 10.1038/s41467-021-27016-x
Reversible and spatiotemporal control of colloidal structure formation
Abstract
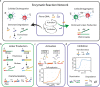
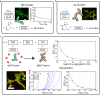
Tuning colloidal structure formation is a powerful approach to building functional materials, as a wide range of optical and viscoelastic properties can be accessed by the choice of individual building blocks and their interactions. Precise control is achieved by DNA specificity, depletion forces, or geometric constraints and results in a variety of complex structures. Due to the lack of control and reversibility of the interactions, an autonomous oscillating system on a mesoscale without external driving was not feasible until now. Here, we show that tunable DNA reaction circuits controlling linker strand concentrations can drive the dynamic and fully reversible assembly of DNA-functionalized micron-sized particles. The versatility of this approach is demonstrated by programming colloidal interactions in sequential and spatial order to obtain an oscillatory structure formation process on a mesoscopic scale. The experimental results represent an approach for the development of active materials by using DNA reaction networks to scale up the dynamic control of colloidal self-organization.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Glotzer SC, Solomon MJ, Kotov NA. Self‐assembly: from nanoscale to microscale colloids. AIChE J. 2004;50:2978–2985. doi: 10.1002/aic.10413. - DOI
-
- Khlebtsov NG, Dykman LA, Krasnov YM, Mel’nikov AG. Light absorption by the clusters of colloidal gold and silver particles formed during slow and fast aggregation. Colloid J. 2000;62:765–779. doi: 10.1023/A:1026643111821. - DOI
-
- Fan, J. A. et al. Self-assembled plasmonic nanoparticle clusters. Science10.1126/science.1187949 (2010). - PubMed
-
- Simmel FC, Schulman R. Self-organizing materials built with DNA. MRS Bull. 2017;42:913–919. doi: 10.1557/mrs.2017.271. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

