EZH2 presents a therapeutic target for neuroendocrine tumors of the small intestine
- PMID: 34815475
- PMCID: PMC8611048
- DOI: 10.1038/s41598-021-02181-7
EZH2 presents a therapeutic target for neuroendocrine tumors of the small intestine
Abstract
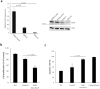
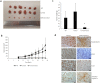
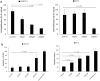
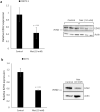
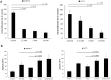
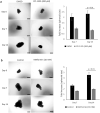
Small intestinal neuroendocrine tumors (SI-NETs) are slow-growing tumors that seem genetically quite stable without highly recurrent mutations, but are epigenetically dysregulated. In contrast to the undetectable expression of the enhancer of zeste homolog 2 (EZH2) histone methyltransferase in the enterochromaffin cells of the small intestine, we found high and differential expression of EZH2 in primary SI-NETs and corresponding metastases. Silencing EZH2 in the SI-NET cell line CNDT2.5 reduced cell proliferation and induced apoptosis. Furthermore, EZH2 knockout inhibited tumor progression in a CNDT2.5 SI-NET xenograft mouse model, and treatment of SI-NET cell lines CNDT2.5 and GOT1 with the EZH2-specific inhibitor CPI-1205 decreased cell viability and promoted apoptosis. Moreover, CPI-1205 treatment reduced migration capacity of CNDT2.5 cells. The EZH2 inhibitor GSK126 also repressed proliferation of CNDT2.5 cells. Recently, metformin has received wide attention as a therapeutic option in diverse cancers. In CNDT2.5 and GOT1 cells, metformin suppressed EZH2 expression, and inhibited cell proliferation. Exposure of GOT1 three-dimensional cell spheroids to CPI-1205 or metformin arrested cell proliferation and decreased spheroid size. These novel findings support a possible role of EZH2 as a candidate oncogene in SI-NETs, and suggest that CPI-1205 and metformin should be further evaluated as therapeutic options for patients with SI-NETs.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

