The von Willebrand factor stamps plasmatic extracellular vesicles from glioblastoma patients
- PMID: 34815502
- PMCID: PMC8611030
- DOI: 10.1038/s41598-021-02254-7
The von Willebrand factor stamps plasmatic extracellular vesicles from glioblastoma patients
Abstract
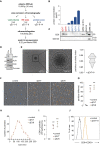
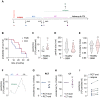
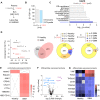
Glioblastoma is a devastating tumor of the central nervous system characterized by a poor survival and an extremely dark prognosis, making its diagnosis, treatment and monitoring highly challenging. Numerous studies have highlighted extracellular vesicles (EVs) as key players of tumor growth, invasiveness and resistance, as they carry and disseminate oncogenic material in the local tumor microenvironment and at distance. However, whether their quality and quantity reflect individual health status and changes in homeostasis is still not fully elucidated. Here, we separated EVs from plasma collected at different time points alongside with the clinical management of GBM patients. Our findings confirm that plasmatic EVs could be separated and characterized with standardized protocols, thereby ensuring the reliability of measuring vesiclemia, i.e. extracellular vesicle concentration in plasma. This unveils that vesiclemia is a dynamic parameter, which could be reflecting tumor burden and/or response to treatments. Further label-free liquid chromatography tandem mass spectrometry unmasks the von Willebrand Factor (VWF) as a selective protein hallmark for GBM-patient EVs. Our data thus support the notion that EVs from GBM patients showed differential protein cargos that can be further surveyed in circulating EVs, together with vesiclemia.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

