Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis
- PMID: 34815503
- PMCID: PMC8611039
- DOI: 10.1038/s41598-021-02321-z
Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis
Abstract
New Coronavirus Disease 2019 (COVID-19) vaccines are available to prevent the ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. We compared the efficacy of new COVID-19 vaccines to prevent symptomatic and severe disease in the adult population and to prevent symptomatic COVID-19 among the elderly. Leading medical databases were searched until August 30, 2021. Published phase 3 randomized controlled trials (RCTs) evaluated efficacy of the vaccine to prevent symptomatic and sever COVID-19 in adults were included. Two reviewers independently evaluated the literature search results and independently extracted summary data. The risk of bias was evaluated using the Cochrane Risk of Bias Assessment Tool. We performed a network meta-analysis (NMA) according to PRISMA-NMA 2015 to pool indirect comparisons between different vaccines regarding their relative efficacy. The primary outcomes were the efficacy of the vaccine against symptomatic COVID-19 in adults (PROSPERO registration number: CRD42021235364). Above 200,000 adult participants from eight phase 3 RCTs were included in NMA, of whom 52% received the intervention (active COVID-19 vaccine). While each of nine vaccines was tested in the unique clinical trial as compared to control, based on indirect comparison, BNT162b2 and mRNA-1273 vaccines were ranked with the highest probability of efficacy against symptomatic COVID-19 (P-scores 0.952 and 0.843, respectively), followed by Gam-COVID-Vac (P-score 0.782), NVX-CoV23730 (P-score 0.700), CoronaVac (P-score 0.570), BN02 (P-score 0.428), WIV04 (P-score 0.327), and Ad26.COV2.S (P-score 0.198). No statistically significant difference was seen in the ability of the vaccines to prevent symptomatic disease in the elderly population. No vaccine was statistically significantly associated with a decreased risk for severe COVID-19 than other vaccines, although mRNA-1273 and Gam-COVID-Vac have the highest P-scores (0.899 and 0.816, respectively), indicating greater protection against severe disease than other vaccines. In our indirect comparison, the BNT162b2 and mRNA-1273 vaccines, which use mRNA technology, were associated with the highest efficacy to prevent symptomatic COVID-19 compared to other vaccines. This finding may have importance when deciding which vaccine to use, together with other important factors as availability of the vaccines, costs, logistics, side effects, and patient acceptability.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
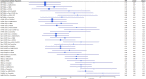
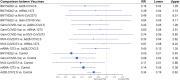
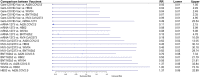
References
-
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. https://covid19.who.int/.
-
- Vaccine Development, Testing, and Regulation | History of Vaccines. https://www.historyofvaccines.org/content/articles/vaccine-development-t....
-
- COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

