Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment
- PMID: 34815799
- PMCID: PMC8581418
- DOI: 10.7150/thno.64229
Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment
Abstract
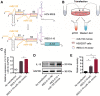
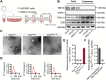
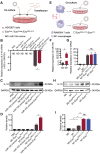
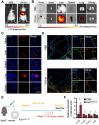
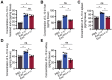
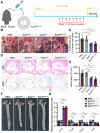
Rationale: Tailored inflammation control is badly needed for the treatment of kinds of inflammatory diseases, such as atherosclerosis. IL-10 is a potent anti-inflammatory cytokine, while systemic and repeated delivery could cause detrimental side-effects due to immune repression. In this study, we have developed a nano-system to deliver inflammation-responsive Il-10 mRNA preferentially into macrophages for tailored inflammation control. Methods:Il-10 was engineered to harbor a modified HCV-IRES (hepatitis C virus internal ribosome entry site), in which the two miR-122 recognition sites were replaced by two miR-155 recognition sites. The translational responsiveness of the engineered mRNA to miR-155 was tested by Western blot or ELISA. Moreover, the engineered Il-10 mRNA was passively encapsulated into exosomes by forced expression in donor cells. Therapeutic effects on atherosclerosis and the systemic leaky expression effects in vivo of the functionalized exosomes were analyzed in ApoE-/- (Apolipoprotein E-deficient) mice. Results: The engineered IRES-Il-10 mRNA could be translationally activated in cells when miR-155 was forced expressed or in M1 polarized macrophages with endogenous miR-155 induced. In addition, the engineered IRES-Il-10 mRNA, when encapsulated into the exosomes, could be efficiently delivered into macrophages and some other cell types in the plaque in ApoE-/- mice. In the recipient cells of the plaque, the encapsulated Il-10 mRNA was functionally translated into protein, with relatively low leaky in other tissues/organs without obvious inflammation. Consistent with the robust Il-10 induction in the plaque, exosome-based delivery of the engineered Il-10 could alleviate the atherosclerosis in ApoE-/- mice. Conclusion: Our study established a potent platform for controlled inflammation control via exosome-based systemic and repeated delivery of engineered Il-10 mRNA, which could be a promising strategy for atherosclerosis treatment.
Keywords: Atherosclerosis; exosomes; inflammation-responsive; interleukin-10; internal ribosome entry site.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–26. - PubMed
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. - PubMed
-
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ. et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;74:e177–e232. - PMC - PubMed
-
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–e143. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

