Electrophysiological study of Arabidopsis ABCB4 and PIN2 auxin transporters: Evidence of auxin activation and interaction enhancing auxin selectivity
- PMID: 34816076
- PMCID: PMC8595762
- DOI: 10.1002/pld3.361
Electrophysiological study of Arabidopsis ABCB4 and PIN2 auxin transporters: Evidence of auxin activation and interaction enhancing auxin selectivity
Abstract
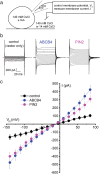
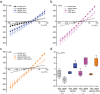
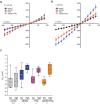
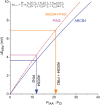
Polar auxin transport through plant tissue strictly requires polarly localized PIN proteins and uniformly distributed ABCB proteins. A functional synergy between the two types of membrane protein where their localizations overlap may create the degree of asymmetric auxin efflux required to produce polar auxin transport. We investigated this possibility by expressing ABCB4 and PIN2 in human embryonic kidney cells and measuring whole-cell ionic currents with the patch-clamp technique and CsCl-based electrolytes. ABCB4 activity was 1.81-fold more selective for Cl- over Cs+ and for PIN2 the value was 2.95. We imposed auxin gradients and determined that ABCB4 and PIN2 were 12-fold more permeable to the auxin anion (IAA-) than Cl-. This measure of the intrinsic selectivity of the transport pathway was 21-fold when ABCB4 and PIN2 were co-expressed. If this increase occurs in plants, it could explain why asymmetric PIN localization is not sufficient to create polar auxin flow. Some form of co-action or synergy between ABCB4 and PIN2 that increases IAA- selectivity at the cell face where both occur may be important. We also found that auxin stimulated ABCB4 activity, which may contribute to a self-reinforcement of auxin transport known as canalization.
© 2021 The Authors. Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The Authors did not report any conflict of interest.
Figures


References
-
- Abas, L. , Benjamins, R. , Malenica, N. , Paciorek, T. , Wiśniewska, J. , Moulinier‐Anzola, J. C. , Sieberer, T. , Friml, J. , & Luschnig, C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin‐efflux facilitator PIN2 are involved in root gravitropism. Nature Cell Biology, 8, 249–256. 10.1038/ncb1369 - DOI - PubMed
-
- Abas, L. , Kolb, M. , Stadlmann, J. , Janacek, D. P. , Lukic, K. , Schwechheimer, C. , Sazanov, L. A. , Mach, L. , Friml, J. , & Hammes, U. Z. (2021). Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proceedings of the National Academy of Sciences of the United States of America, 118(1), e2020857118. - PMC - PubMed
-
- Band, L. R. , Wells, D. M. , Fozard, J. A. , Ghetiu, T. , French, A. P. , Pound, M. P. , Wilson, M. H. , Yu, L. , Li, W. , Hijazi, H. I. , Oh, J. , Pearce, S. P. , Perez‐Amador, M. A. , Yun, J. , Kramer, E. , Alonso, J. M. , Godin, C. , Vernoux, T. , Hodgman, T. C. , … Bennett, M. J. (2014). Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell, 26, 862–875. 10.1105/tpc.113.119495 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

