Lysosomal dysfunction impairs mitochondrial quality control and is associated with neurodegeneration in TBCK encephaloneuronopathy
- PMID: 34816123
- PMCID: PMC8603245
- DOI: 10.1093/braincomms/fcab215
Lysosomal dysfunction impairs mitochondrial quality control and is associated with neurodegeneration in TBCK encephaloneuronopathy
Abstract
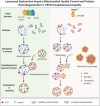
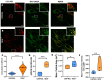
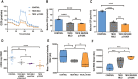
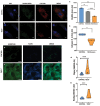
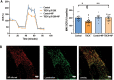
Biallelic variants in the TBCK gene cause intellectual disability with remarkable clinical variability, ranging from static encephalopathy to progressive neurodegeneration (TBCK-Encephaloneuronopathy). The biological factors underlying variable disease penetrance remain unknown. Since previous studies had suggested aberrant autophagy, we tested whether mitophagy and mitochondrial function are altered in TBCK -/- fibroblasts derived from patients exhibiting variable clinical severity. Our data show significant accumulation of mitophagosomes, reduced mitochondrial respiratory capacity and mitochondrial DNA content, suggesting impaired mitochondrial quality control. Furthermore, the degree of mitochondrial dysfunction correlates with a neurodegenerative clinical course. Since mitophagy ultimately depends on lysosomal degradation, we also examined lysosomal function. Our data show that lysosomal proteolytic function is significantly reduced in TBCK -/- fibroblasts. Moreover, acidifying lysosomal nanoparticles rescue the mitochondrial respiratory defects in fibroblasts, suggesting impaired mitochondrial quality control secondary to lysosomal dysfunction. Our data provide insight into the disease mechanisms of TBCK Encephaloneuronopathy and the potential relevance of mitochondrial function as a biomarker beyond primary mitochondrial disorders. It also supports the benefit of lysosomal acidification strategies for disorders of impaired lysosomal degradation affecting mitochondrial quality control.
Keywords: intellectual disability; lysosome; mitochondria; mitophagy; neurodegeneration.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Menzies FM, Fleming A, Rubinsztein DC.. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci. 2015;16(6):345–357. - PubMed
-
- Whyte LS, Lau AA, Hemsley KM, Hopwood JJ, Sargeant TJ.. Endo-lysosomal and autophagic dysfunction: A driving factor in Alzheimer's disease? J Neurochem. 2017;140(5):703–717. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
