Efficacy and Safety of HLX03, an Adalimumab Biosimilar, in Patients with Moderate-to-Severe Plaque Psoriasis: A Randomized, Double-Blind, Phase III Study
- PMID: 34816373
- PMCID: PMC8799567
- DOI: 10.1007/s12325-021-01899-0
Efficacy and Safety of HLX03, an Adalimumab Biosimilar, in Patients with Moderate-to-Severe Plaque Psoriasis: A Randomized, Double-Blind, Phase III Study
Abstract
Introduction: Adalimumab has been used successfully in the treatment of psoriasis. The objective of the study is to compare the efficacy, safety, and immunogenicity between HLX03, an adalimumab biosimilar, and adalimumab in Chinese patients with moderate-to-severe plaque psoriasis.
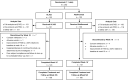
Methods: In this double-blind, active-controlled, parallel-group study, 262 patients with moderate-to-severe plaque psoriasis were randomized (1:1) to receive HLX03 or adalimumab (80 mg at week 1, 40 mg at week 2, and then 40 mg every 2 weeks) for 48 weeks. The primary endpoint was improvement in Psoriasis Area and Severity Index (PASI) score at week 16 comparing to baseline. Equivalence was demonstrated if 95% confidence interval (CI) of the between group difference fell within the equivalence margins of ± 15%. Other efficacy endpoints, safety and immunogenicity were also evaluated.
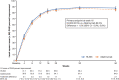
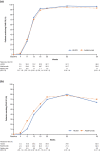
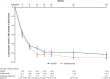
Results: In the full analysis set, PASI improvements at week 16 was 83.5% (n = 131) in the HLX03 group and 82.0% (n = 130) in the adalimumab group, with a least-square-mean difference of 1.5% (95% CI - 3.9% to 6.8%). There were no significant between-group differences in all secondary efficacy analyses including proportion of patients achieving ≥ 75% improvement from baseline PASI (PASI 75), physician global assessment (PGA) 0/1 (clear or almost clear) and change in dermatology life quality index (DLQI) score. The incidences of adverse events and the proportion of patients with antidrug antibodies were also comparable between the two treatment groups.
Conclusion: HLX03 demonstrated equivalent efficacy, similar safety and immunogenicity to reference adalimumab, supporting its development as an alternative treatment for patients with plaque psoriasis in China.
Clinical trial registration: Chinadrugtrials.org.cn, CTR20171123 (November 27, 2017); ClinicalTrials.gov, NCT03316781 (October 20, 2017).
Keywords: Adalimumab; Biosimilar; Plaque psoriasis; TNFα inhibitor.
Plain language summary
Plaque psoriasis is a chronic, autoimmune, inflammatory skin disease associated with significant morbidity and reduced quality of life. In China, the prevalence of plaque psoriasis increased four-fold between 1987 and 2012. Adalimumab is a biologic antibody used to treat plaque psoriasis globally. However, high treatment costs remain as a significant barrier to adalimumab therapy. Therefore, HLX03 has been developed as an adalimumab (Humira®) biosimilar, which is almost identical to the licensed reference adalimumab, but less expensive and more accessible to patients. In this randomized clinical trial, the efficacy (ability of a drug to produce the desired treatment effects), safety, and immunogenicity (ability of a drug to induce immune response which would affect its efficacy and safety) of HLX03 were compared with the reference adalimumab in Chinese patients with moderate-to-severe plaque psoriasis. Efficacy was evaluated by comparing the changes in severity and extent of disease using Psoriasis Area and Severity Index score between treatment initiation and week 16. Safety was monitored by adverse events, laboratory tests and vital signs. Immunogenicity was assessed by the incidence of antidrug antibodies. Among the 262 randomized patients, 131 received HLX03 and 130 received adalimumab. Both groups reported similar improvements in Psoriasis Area and Severity Index scores (between-group difference fell within the prespecified equivalence margins), and also in other efficacy evaluations. Additionally, the two treatment groups showed similar safety and immunogenicity profiles. In summary, HLX03 demonstrated equivalent efficacy to adalimumab, validating it as an alternative treatment for patients with plaque psoriasis in China.
© 2021. The Author(s).
Figures
References
-
- AbbVie Inc. Humira® prescribing information. https://www.rxabbvie.com/pdf/humira.pdf. Accessed 24 Aug 2020.
-
- AbbVie Inc. Humira® Summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/humira-epar-p.... Accessed 19 Aug 2020.
-
- National Medical Products Administration China. NMPA guidelines for clinical trials of adalimumab injection biosimilars. 2020. https://www.nmpa.gov.cn/directory/web/nmpa/images/1596531455261017949.pdf. Accessed 24 Aug 2020.
-
- AbbVie Inc. Chinese-licensed adalimumab injection instructions. 2020. https://xiumeile.com/index.php/shuomingshu.html. Accessed 24 Aug 2020.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

