Brain Kynurenine Pathway and Functional Outcome of Rats Resuscitated From Cardiac Arrest
- PMID: 34816736
- PMCID: PMC9075408
- DOI: 10.1161/JAHA.121.021071
Brain Kynurenine Pathway and Functional Outcome of Rats Resuscitated From Cardiac Arrest
Abstract
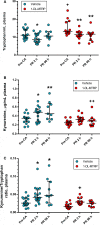
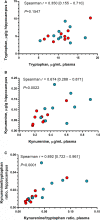
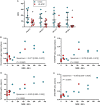
Background Brain injury and neurological deficit are consequences of cardiac arrest (CA), leading to high morbidity and mortality. Peripheral activation of the kynurenine pathway (KP), the main catabolic route of tryptophan metabolized at first into kynurenine, predicts poor neurological outcome in patients resuscitated after out-of-hospital CA. Here, we investigated KP activation in hippocampus and plasma of rats resuscitated from CA, evaluating the effect of KP modulation in preventing CA-induced neurological deficit. Methods and Results Early KP activation was first demonstrated in 28 rats subjected to electrically induced CA followed by cardiopulmonary resuscitation. Hippocampal levels of the neuroactive metabolites kynurenine, 3-hydroxy-anthranilic acid, and kynurenic acid were higher 2 hours after CA, as in plasma. Further, 36 rats were randomized to receive the inhibitor of the first step of KP, 1-methyl-DL-tryptophan, or vehicle, before CA. No differences were observed in hemodynamics and myocardial function. The CA-induced KP activation, sustained up to 96 hours in hippocampus (and plasma) of vehicle-treated rats, was counteracted by the inhibitor as indicated by lower hippocampal (and plasmatic) kynurenine/tryptophan ratio and kynurenine levels. 1-Methyl-DL-tryptophan reduced the CA-induced neurological deficits, with a significant correlation between the neurological score and the individual kynurenine levels, as well as the kynurenine/tryptophan ratio, in plasma and hippocampus. Conclusions These data demonstrate the CA-induced lasting activation of the first step of the KP in hippocampus, showing that this activation was involved in the evolving neurological deficit. The degree of peripheral activation of KP may predict neurological function after CA.
Keywords: cardiac arrest; cardiopulmonary resuscitation; indoleamine 2,3‐deoxygenase; kynurenine pathway; neurological deficit.
Figures


References
-
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A scientific statement from the international liaison committee on resuscitation; the american heart association emergency cardiovascular care committee; the council on cardiovascular surgery and anesthesia; the council on cardiopulmonary, perioperative, and critical care; the council on clinical cardiology; the council on stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017 - DOI - PubMed
-
- Lilja G, Nielsen N, Bro‐Jeppesen J, Dunford H, Friberg H, Hofgren C, Horn J, Insorsi A, Kjaergaard J, Nilsson F, et al. Return to work and participation in society after out‐of‐hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2018;11:e003566. doi: 10.1161/CIRCOUTCOMES.117.003566 - DOI - PubMed

