Understanding the Effects of Binders in Gas Sorption and Acidity of Aluminium Fumarate Extrudates
- PMID: 34817102
- PMCID: PMC9299853
- DOI: 10.1002/chem.202103420
Understanding the Effects of Binders in Gas Sorption and Acidity of Aluminium Fumarate Extrudates
Abstract
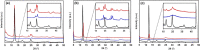
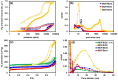
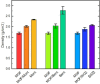
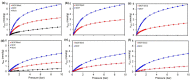
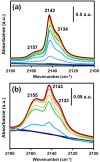
Understanding the impact of shaping processes on solid adsorbents is critical for the implementation of MOFs in industrial separation processes or as catalytic materials. Production of MOF-containing shaped particles is typically associated with loss of porosity and modification of acid sites, two phenomena that affect their performance. Herein, we report a detailed study on how extrusion affects the crystallinity, porosity, and acidity of the aluminium fumarate MOF with clays or SiO2 gel binders. Thorough characterization showed that the clay binders confer the extrudates a good mechanical robustness at the expense of porosity, while silica gel shows an opposite trend. The CO2 selectivity towards CH4 , of interest for natural gas separation processes, is maintained upon the extrusion process. Moreover, probe FTIR spectroscopy revealed no major changes in the types of acid sites. This study highlights that these abundant and inexpensive clay materials may be used for scaling MOFs as active adsorbents.
Keywords: CO2/CH4; acidity; aluminium fumarate; metal-organic frameworks (MOFs); solids extrusion.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Furukawa H., Cordova K. E., O'Keeffe M., Yaghi O. M., Science 2013, 341, 6149. - PubMed
-
- Zhou H.-C., Long J. R., Yaghi O. M., Chem. Rev. 2012, 112, 673–674. - PubMed
-
- Tagliabue M., Rizzo C., Millini R., Dietzel P. D. C., Blom R., Zanardi S., J. Porous Mater. 2011, 18, 289–296.
-
- Castro-Gonzales L., Kimmerle K., Schippert E., Fickinger D., ChemistrySelect 2016, 1, 2834–2841.
-
- Chanut N., Wiersum A. D., Lee U. H., Hwang Y. K., Ragon F., Chevreau H., Bourrelly S., Kuchta B., Chang J.-S., Serre C., Llewellyn P. L., Eur. J. Inorg. Chem. 2016, 4416–4423.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

