Analysis of Glycosylation and Disulfide Bonding of Wild-Type SARS-CoV-2 Spike Glycoprotein
- PMID: 34817202
- PMCID: PMC8827021
- DOI: 10.1128/JVI.01626-21
Analysis of Glycosylation and Disulfide Bonding of Wild-Type SARS-CoV-2 Spike Glycoprotein
Abstract
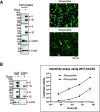
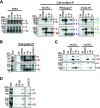
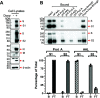
The SARS-CoV-2 coronavirus, the etiologic agent of COVID-19, uses its spike (S) glycoprotein anchored in the viral membrane to enter host cells. The S glycoprotein is the major target for neutralizing antibodies elicited by natural infection and by vaccines. Approximately 35% of the SARS-CoV-2 S glycoprotein consists of carbohydrate, which can influence virus infectivity and susceptibility to antibody inhibition. We found that virus-like particles produced by coexpression of SARS-CoV-2 S, M, E, and N proteins contained spike glycoproteins that were extensively modified by complex carbohydrates. We used a fucose-selective lectin to purify the Golgi-modified fraction of a wild-type SARS-CoV-2 S glycoprotein trimer and determined its glycosylation and disulfide bond profile. Compared with soluble or solubilized S glycoproteins modified to prevent proteolytic cleavage and to retain a prefusion conformation, more of the wild-type S glycoprotein N-linked glycans are processed to complex forms. Even Asn 234, a significant percentage of which is decorated by high-mannose glycans on other characterized S trimer preparations, is predominantly modified in the Golgi compartment by processed glycans. Three incompletely occupied sites of O-linked glycosylation were detected. Viruses pseudotyped with natural variants of the serine/threonine residues implicated in O-linked glycosylation were generally infectious and exhibited sensitivity to neutralization by soluble ACE2 and convalescent antisera comparable to that of the wild-type virus. Unlike other natural cysteine variants, a Cys15Phe (C15F) mutant retained partial, but unstable, infectivity. These findings enhance our understanding of the Golgi processing of the native SARS-CoV-2 S glycoprotein carbohydrates and could assist the design of interventions. IMPORTANCE The SARS-CoV-2 coronavirus, which causes COVID-19, uses its spike glycoprotein to enter host cells. The viral spike glycoprotein is the main target of host neutralizing antibodies that help to control SARS-CoV-2 infection and are important for the protection provided by vaccines. The SARS-CoV-2 spike glycoprotein consists of a trimer of two subunits covered with a coat of carbohydrates (sugars). Here, we describe the disulfide bonds that assist the SARS-CoV-2 spike glycoprotein to assume the correct shape and the composition of the sugar moieties on the glycoprotein surface. We also evaluate the consequences of natural virus variation in O-linked sugar addition and in the cysteine residues involved in disulfide bond formation. This information can expedite the improvement of vaccines and therapies for COVID-19.
Keywords: COVID-19; Golgi; SARS-CoV-2; coronavirus; disulfide; glycosylation; membrane protein; spike glycoprotein; viral protein; virus entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Update of
-
Analysis of glycosylation and disulfide bonding of wild-type SARS-CoV-2 spike glycoprotein.bioRxiv [Preprint]. 2021 Apr 1:2021.04.01.438120. doi: 10.1101/2021.04.01.438120. bioRxiv. 2021. Update in: J Virol. 2022 Feb 9;96(3):e0162621. doi: 10.1128/JVI.01626-21. PMID: 33821278 Free PMC article. Updated. Preprint.
Similar articles
-
Analysis of glycosylation and disulfide bonding of wild-type SARS-CoV-2 spike glycoprotein.bioRxiv [Preprint]. 2021 Apr 1:2021.04.01.438120. doi: 10.1101/2021.04.01.438120. bioRxiv. 2021. Update in: J Virol. 2022 Feb 9;96(3):e0162621. doi: 10.1128/JVI.01626-21. PMID: 33821278 Free PMC article. Updated. Preprint.
-
Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration.Elife. 2020 Oct 26;9:e61552. doi: 10.7554/eLife.61552. Elife. 2020. PMID: 33103998 Free PMC article.
-
Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants.Elife. 2020 Oct 28;9:e61312. doi: 10.7554/eLife.61312. Elife. 2020. PMID: 33112236 Free PMC article.
-
Glycosylation of SARS-CoV-2: structural and functional insights.Anal Bioanal Chem. 2021 Dec;413(29):7179-7193. doi: 10.1007/s00216-021-03499-x. Epub 2021 Jul 7. Anal Bioanal Chem. 2021. PMID: 34235568 Free PMC article. Review.
-
Structural basis of severe acute respiratory syndrome coronavirus 2 infection.Curr Opin HIV AIDS. 2021 Jan;16(1):74-81. doi: 10.1097/COH.0000000000000658. Curr Opin HIV AIDS. 2021. PMID: 33186231 Review.
Cited by
-
SARS-CoV-2 Spike Protein Mutation at Cysteine-488 Impairs Its Golgi Localization and Intracellular S1/S2 Processing.Int J Mol Sci. 2022 Dec 13;23(24):15834. doi: 10.3390/ijms232415834. Int J Mol Sci. 2022. PMID: 36555473 Free PMC article.
-
Enhanced Surface Accessibility of SARS-CoV-2 Omicron Spike Protein Due to an Altered Glycosylation Profile.ACS Infect Dis. 2024 Jun 14;10(6):2032-2046. doi: 10.1021/acsinfecdis.4c00015. Epub 2024 May 10. ACS Infect Dis. 2024. PMID: 38728322 Free PMC article.
-
Proteomics-based mass spectrometry profiling of SARS-CoV-2 infection from human nasopharyngeal samples.Mass Spectrom Rev. 2024 Jan-Feb;43(1):193-229. doi: 10.1002/mas.21813. Epub 2022 Sep 29. Mass Spectrom Rev. 2024. PMID: 36177493 Free PMC article. Review.
-
Identification and differential usage of a host metalloproteinase entry pathway by SARS-CoV-2 Delta and Omicron.iScience. 2022 Nov 18;25(11):105316. doi: 10.1016/j.isci.2022.105316. Epub 2022 Oct 10. iScience. 2022. PMID: 36254158 Free PMC article.
-
Multifaceted membrane binding head of the SARS-CoV-2 spike protein.Curr Res Struct Biol. 2022 May 16;4:146-157. doi: 10.1016/j.crstbi.2022.05.001. eCollection 2022. Curr Res Struct Biol. 2022. PMID: 35602928 Free PMC article.
References
-
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. 10.1056/NEJMoa2001316. - DOI - PMC - PubMed
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. 10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

