Topiramate for juvenile myoclonic epilepsy
- PMID: 34817852
- PMCID: PMC8612308
- DOI: 10.1002/14651858.CD010008.pub5
Topiramate for juvenile myoclonic epilepsy
Abstract
Background: Topiramate is a newer broad-spectrum antiepileptic drug (AED). Some studies have shown the benefits of topiramate in the treatment of juvenile myoclonic epilepsy (JME). However, there are no current systematic reviews to determine the efficacy and tolerability of topiramate in people with JME. This is an update of a Cochrane Review first published in 2015, and last updated in 2019.
Objectives: To evaluate the efficacy and tolerability of topiramate in the treatment of JME.
Search methods: For the latest update, we searched the Cochrane Register of Studies (CRS Web) on 26 August 2021, and MEDLINE (Ovid 1946 to 26 August 2021). CRS Web includes randomized or quasi-randomized controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups, including Cochrane Epilepsy.
Selection criteria: We included randomized controlled trials (RCTs) investigating topiramate versus placebo or other AED treatment for people with JME, with the outcomes of proportion of responders and proportion of participants experiencing adverse events (AEs).
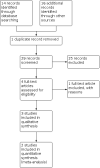
Data collection and analysis: Two review authors independently screened the titles and abstracts of identified records, selected studies for inclusion, extracted data, cross-checked the data for accuracy and assessed the methodological quality of the studies.
Main results: We included three studies with a total of 83 participants. For efficacy, a greater proportion of participants in the topiramate group had a 50% or greater reduction in primarily generalized tonic-clonic seizures (PGTCS), compared with participants in the placebo group (RR 4.00, 95% CI 1.08 to 14.75; 1 study, 22 participants; very low-certainty evidence). There were no significant differences between topiramate and valproate for participants responding with a 50% or greater reduction in myoclonic seizures (RR 0.88, 95% CI 0.67 to 1.15; one study, 23 participants; very-low certainty evidence) or in PGTCS (RR 1.22, 95% CI 0.68 to 2.21; one study, 16 participants, very-low certainty evidence), or participants becoming seizure-free (RR 1.13, 95% CI 0.61 to 2.11; one study, 27 participants; very-low certainty evidence). Concerning tolerability, we ranked AEs associated with topiramate as moderate to severe, while we ranked 59% of AEs linked to valproate as severe complaints (2 studies, 61 participants; very low-certainty evidence). Moreover, systemic toxicity scores were higher in the valproate group than the topiramate group. Overall we judged all three studies to be at high risk of attrition bias and at unclear risk of reporting bias. We judged the studies to be at low to unclear risk of bias for the remaining domains (selection bias, performance bias, detection bias and other bias). We judged the overall certainty of the evidence for the outcomes as very low using the GRADE approach.
Authors' conclusions: We have found no new studies since the last version of this review was published in 2019. This review does not provide sufficient evidence to support topiramate for the treatment of people with JME. Based on the current limited available data, topiramate seems to be better tolerated than valproate, but has no clear benefits over valproate in terms of efficacy. Well-designed, double-blind RCTs with large samples are required to test the efficacy and tolerability of topiramate in people with JME.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Jia Liu: none known
Yao‐Jun Tai: none known
Lu‐Ning Wang: none known
Figures





























Update of
-
Topiramate for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2019 Jan 28;1(1):CD010008. doi: 10.1002/14651858.CD010008.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 24;11:CD010008. doi: 10.1002/14651858.CD010008.pub5. PMID: 30687937 Free PMC article. Updated.
Similar articles
-
Topiramate monotherapy for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2017 Apr 23;4(4):CD010008. doi: 10.1002/14651858.CD010008.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jan 28;1:CD010008. doi: 10.1002/14651858.CD010008.pub4. PMID: 28434203 Free PMC article. Updated.
-
Topiramate monotherapy for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2015 Dec 23;(12):CD010008. doi: 10.1002/14651858.CD010008.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Apr 23;4:CD010008. doi: 10.1002/14651858.CD010008.pub3. PMID: 26695884 Updated.
-
Clonazepam monotherapy for treating people with newly diagnosed epilepsy.Cochrane Database Syst Rev. 2022 Feb 21;2(2):CD013028. doi: 10.1002/14651858.CD013028.pub3. Cochrane Database Syst Rev. 2022. PMID: 35187637 Free PMC article.
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
-
Pregabalin add-on for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2022 Mar 29;3(3):CD005612. doi: 10.1002/14651858.CD005612.pub5. Cochrane Database Syst Rev. 2022. PMID: 35349176 Free PMC article.
Cited by
-
Altered effective connectivity of the default mode network in juvenile myoclonic epilepsy.Cogn Neurodyn. 2024 Aug;18(4):1549-1561. doi: 10.1007/s11571-023-09994-4. Epub 2023 Jul 31. Cogn Neurodyn. 2024. PMID: 39104702 Free PMC article.
-
Exploring the role of interleukin-6 receptor blockade in epilepsy and associated neuropsychiatric conditions through a mendelian randomization study.World J Psychiatry. 2024 Aug 19;14(8):1244-1253. doi: 10.5498/wjp.v14.i8.1244. eCollection 2024 Aug 19. World J Psychiatry. 2024. PMID: 39165549 Free PMC article.
-
Antiseizure medications for idiopathic generalized epilepsies: a systematic review and network meta-analysis.J Neurol. 2023 Oct;270(10):4713-4728. doi: 10.1007/s00415-023-11834-8. Epub 2023 Jun 28. J Neurol. 2023. PMID: 37378757 Free PMC article.
References
References to studies included in this review
Biton 2005 {published data only}
-
- Biton V, Bourgeois BF, YTC/YTCE Study Investigators. Topiramate in patients with juvenile myoclonic epilepsy. Archives of Neurology 2005;62(11):1705-8. [PMID: ] - PubMed
Levisohn 2007 {published data only}
-
- Levisohn PM, Holland KD. Topiramate or valproate in patients with juvenile myoclonic epilepsy: a randomized open-label comparison. Epilepsy & Behavior 2007;10(4):547-52. [PMID: ] - PubMed
Park 2013 {published data only}
-
- Park KM, Kim SH, Nho SK, Shin KJ, Park J, Ha SY, et al. A randomized open-label observational study to compare the efficacy and tolerability between topiramate and valproate in juvenile myoclonic epilepsy. Journal of Clinical Neuroscience 2013;20(8):1079-82. [PMID: ] - PubMed
References to studies excluded from this review
Sousa 2005 {published data only}
-
- Sousa Pda S, Araújo Filho GM, Garzon E, Sakamoto AC, Yacubian EM. Topiramate for the treatment of juvenile myoclonic epilepsy. Arquivos de Neuro-psiquiatria 2005;63(3B):733-7. [PMID: ] - PubMed
Additional references
Alfradique 2007
-
- Alfradique I, Vasconcelos MM. Juvenile myoclonic epilepsy. Arquivos de Neuro-psiquiatria 2007;65(4B):1266-71. [PMID: ] - PubMed
Biton 1999
-
- Biton V, Montouris GD, Ritter F, Riviello JJ, Reife R, Lim P, et al. A randomized, placebo-controlled study of topiramate in primary generalized tonic-clonic seizures. Neurology 1999;52(7):1330-7. [PMID: ] - PubMed
Calleja 2001
-
- Calleja S, Salas-Puig J, Ribacoba R, Lahoz CH. Evolution of juvenile myoclonic epilepsy treated from the outset with sodium valproate. Seizure 2001;10(6):424-7. [PMID: ] - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [PMID: ] - PubMed
Guberman 2002
-
- Guberman A, Neto W, Gassmann-Mayer C, EPAJ-119 Study Group. Low-dose topiramate in adults with treatment-resistant partial-onset seizures. Acta Neurologica Scandinavica 2002;106(4):183-9. [PMID: ] - PubMed
Hanaya 1998
-
- Hanaya R, Sasa M, Ujihara H, Ishihara K, Serikawa T, Iida K, et al. Suppression by topiramate of epileptiform burst discharges in hippocampal CA3 neurons of spontaneously epileptic rat in vitro. Brain Research 1998;789(2):274-82. [PMID: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Jallon 2005
-
- Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia 2005;46(Suppl 9):10-4. [PMID: ] - PubMed
Janz 1985
-
- Janz D. Epilepsy with impulsive petit mal (juvenile myoclonic epilepsy). Acta Neurologica Scandinavica 1985;72(5):449-59. [PMID: ] - PubMed
Kirkham 2010
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [PMID: ] - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Montouris 2009
-
- Montouris G, Abou-Khalil B. The first line of therapy in a girl with juvenile myoclonic epilepsy: should it be valproate or a new agent? Epilepsia 2009;50(Suppl 8):16-20. [PMID: ] - PubMed
Panayiotopoulos 1991
-
- Panayiotopoulos CP, Tahan R, Obeid T. Juvenile myoclonic epilepsy: factors of error involved in the diagnosis and treatment. Epilepsia 1991;32(5):672-6. [PMID: ] - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sachdeo 1999
-
- Sachdeo RC, Glauser TA, Ritter F, Reife R, Lim P, Pledger G, Topiramate YL Study Group. A double-blind, randomized trial of topiramate in Lennox-Gastaut syndrome. Neurology 1999;52(9):1882-7. [PMID: ] - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Serafini 2019
-
- Serafini A, Gerard E, Genton E, Crespel A, Gelisse P. Treatment of juvenile myoclonic epilepsy in patients of child-bearing potential. CNS Drugs 2019;33(3):195-208. - PubMed
Sharpe 2008
-
- Sharpe DV, Patel AD, Abou-Khalil B, Fenichel GM. Levetiracetam monotherapy in juvenile myoclonic epilepsy. Seizure 2008;17(1):64-8. [PMID: ] - PubMed
White 2000
-
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia 2000;41(Suppl 1):17-20. [PMID: ] - PubMed
Zona 1997
-
- Zona C, Ciotti MT, Avoli M. Topiramate attenuates voltage-gated sodium currents in rat cerebellar granule cells. Neuroscience Letters 1997;231(3):123-6. [PMID: ] - PubMed
References to other published versions of this review
Liu 2012
Liu 2015
Liu 2017
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

