Sequential immunization of macaques elicits heterologous neutralizing antibodies targeting the V3-glycan patch of HIV-1 Env
- PMID: 34818054
- PMCID: PMC8932345
- DOI: 10.1126/scitranslmed.abk1533
Sequential immunization of macaques elicits heterologous neutralizing antibodies targeting the V3-glycan patch of HIV-1 Env
Abstract
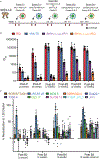
Broadly neutralizing antibodies (bNAbs) against HIV-1 develop after prolonged virus and antibody coevolution. Previous studies showed that sequential immunization with a V3-glycan patch germline-targeting HIV-1 envelope trimer (Env) followed by variant Envs can reproduce this process in mice carrying V3-glycan bNAb precursor B cells. However, eliciting bNAbs in animals with polyclonal antibody repertoires is more difficult. We used a V3-glycan immunogen multimerized on virus-like particles (VLPs), followed by boosting with increasingly native-like Env-VLPs, to elicit heterologous neutralizing antibodies in nonhuman primates (NHPs). Structures of antibody/Env complexes after prime and boost vaccinations demonstrated target epitope recognition with apparent maturation to accommodate glycans. However, we also observed increasing off-target antibodies with boosting. Eight vaccinated NHPs were subsequently challenged with simian-human immunodeficiency virus (SHIV), and seven of eight animals became infected. The single NHP that remained uninfected after viral challenge exhibited one of the lowest neutralization titers against the challenge virus. These results demonstrate that more potent heterologous neutralization resulting from sequential immunization is necessary for protection in this animal model. Thus, improved prime-boost regimens to increase bNAb potency and stimulate other immune protection mechanisms are essential for developing anti–HIV-1 vaccines.
Conflict of interest statement
Figures


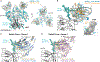


References
-
- Baba TW et al. , Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 6, 200–206 (2000). - PubMed
-
- Eichberg JW, Murthy KK, Ward RH, Prince AM, Prevention of HIV infection by passive immunization with HIVIG or CD4-IgG. AIDS Res Hum Retroviruses 8, 1515 (1992). - PubMed
-
- Emini EA et al. , Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355, 728–730 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

