Oxidative damage and delayed replication allow viable Mycobacterium tuberculosis to go undetected
- PMID: 34818059
- PMCID: PMC8903021
- DOI: 10.1126/scitranslmed.abg2612
Oxidative damage and delayed replication allow viable Mycobacterium tuberculosis to go undetected
Abstract
“Viable but nonculturable” states of bacteria pose challenges for environmental and clinical microbiology, but their biological mechanisms remain obscure. Mycobacterium tuberculosis (Mtb), the leading cause of death from infection until the coronavirus disease 2019 pandemic, affords a notable example of this phenotype. Mtb can enter into a “differentially detectable” (DD) state associated with phenotypic antimicrobial resistance. In this state, Mtb cells are viable but undetectable as colony-forming units. We found that Mtb cells enter the DD state when they undergo sublethal oxidative stress that damages their DNA, proteins, and lipids. In addition, their replication process is delayed, allowing time for repair. Mycobacterium bovis and its derivative, BCG, fail to enter the DD state under similar conditions. These findings have implications for tuberculosis latency, detection, relapse, treatment monitoring, and development of regimens that overcome phenotypic antimicrobial resistance.
Conflict of interest statement
Declaration of Interests
CN serves on advisory bodies for Tres Cantos Open Lab Foundation, Tri-Institutional Therapeutics Discovery Institute, Bridge Medicines, Pfizer External Sciences and Innovation, and Leap Therapeutics. All other authors declare no conflicts of interest related to this work.
Figures
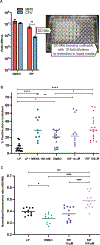


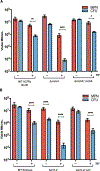


References
-
- Global tuberculosis report 2020 (World Health Organization, Geneva, Switzerland, 2020).
-
- Merle CS, Fielding K, Sow OB, Gninafon M, Lo MB, Mthiyane T, Odhiambo J, Amukoye E, Bah B, Kassa F, N’Diaye A, Rustomjee R, de Jong BC, Horton J, Perronne C, Sismanidis C, Lapujade O, Olliaro PL, Lienhardt C, A four-month gatifloxacin-containing regimen for treating tuberculosis. New England Journal of Medicine 371, 1588–1598 (2014). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

