Evaluation of the impact of a clinical pharmacist on a specialty neurology clinic's adherence to recommended laboratory test monitoring
- PMID: 34818084
- PMCID: PMC10391173
- DOI: 10.18553/jmcp.2021.27.12.1664
Evaluation of the impact of a clinical pharmacist on a specialty neurology clinic's adherence to recommended laboratory test monitoring
Abstract
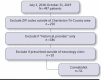
BACKGROUND: Pharmacists can have a significant effect on the specialty ambulatory care setting. Specialty medications are potentially high risk and may require frequent laboratory test monitoring to assess for therapy-associated adverse effects. Pharmacists may work under collaborative drug therapy management agreements that allow for the ordering and assessment of recommended laboratory tests in order to optimize safe and effective medication use. The impact of pharmacists on clinic adherence to recommended laboratory test monitoring has yet to be described in the literature. OBJECTIVE: To describe the impact of a specialty clinical pharmacist on neurology clinic adherence to manufacturer-recommended laboratory test monitoring. METHODS: This study was a retrospective chart review at a single academic medical center for the period between July 1, 2014, and April 30, 2020, comparing laboratory test monitoring adherence before (prepharmacist) and after (post-pharmacist) incorporation of a pharmacist into a neurology clinic. Patients were included if they lived in the Tri-County Area of Charleston, South Carolina, and received a prescription for dalfampridine, dimethyl fumarate, fingolimod, teriflunomide, or cannabidiol that was prescribed by a neurology clinic provider at the Medical University of South Carolina. Chart review was conducted to assess clinic adherence with manufacturer-recommended laboratory test monitoring. Laboratory test monitoring was considered adherent if obtained within 6 months before or on the date of prescription order. Descriptive statistics were calculated for all variables, and adherence rates were compared using chi-square or Fisher's exact tests. RESULTS: For dalfampridine, dimethyl fumarate, fingolimod, and teriflunomide, there were 123 patients and 78 patients in the pre- and post-pharmacist groups, respectively. There were 51 patients in the cannabidiol group. Clinic adherence to laboratory test monitoring improved in the post-pharmacist group for every monitoring point, with statistically significant improvement in "hepatic function tests every 6-9 months" (P = 0.005), "CBC every 6-9 months" (P = 0.01), and "VZV IgG titer at baseline" (P = 0.005) for patients taking fingolimod. CONCLUSIONS: Our study demonstrated improved adherence to manufacturer-recommended laboratory test monitoring after a specialty clinical pharmacist was incorporated into a multidisciplinary neurology clinic. DISCLOSURES: No funding supported this study. The authors have nothing to disclose.
Conflict of interest statement
No funding supported this study. The authors have nothing to disclose.
Figures
Similar articles
-
Improvement in safety monitoring of biologic response modifiers after the implementation of clinical care guidelines by a specialty.J Manag Care Pharm. 2013 Jan-Feb;19(1):49-67. doi: 10.18553/jmcp.2013.19.1.49. J Manag Care Pharm. 2013. PMID: 23383700 Free PMC article.
-
Evaluation of a pharmacist-managed amiodarone monitoring program.J Manag Care Pharm. 2011 Sep;17(7):513-22. doi: 10.18553/jmcp.2011.17.7.513. J Manag Care Pharm. 2011. PMID: 21870892 Free PMC article.
-
The impact of a pharmacist-led oral anticancer clinic on medication adherence and laboratory monitoring.J Oncol Pharm Pract. 2023 Dec;29(8):1921-1927. doi: 10.1177/10781552231159870. Epub 2023 Jun 23. J Oncol Pharm Pract. 2023. PMID: 37350157
-
Initial experience with antiarrhythmic medication monitoring by clinical pharmacists in an outpatient setting: a retrospective review.Clin Ther. 2009 Jun;31(6):1209-18. doi: 10.1016/j.clinthera.2009.06.014. Clin Ther. 2009. PMID: 19695388
-
Advancing Patient Care Through Specialty Pharmacy Services in an Academic Health System.J Manag Care Spec Pharm. 2017 Aug;23(8):815-820. doi: 10.18553/jmcp.2017.23.8.815. J Manag Care Spec Pharm. 2017. PMID: 28737983 Free PMC article.
References
-
- American Pharmacists Association. Specialty pharmacy. Accessed March 2, 2020. https://www.pharmacist.com/Practice/Patient-Care-Services/Specialty
-
- Habibi M, Kuttab HM. Management of multiple sclerosis and the integration of related specialty pharmacy programs within health systems. Am J Health Syst Pharm. 2016;73(11):811-19. - PubMed
-
- Ampyra. Prescribing information. Acorda Therapeutics, Inc.; 2019. Accessed October 18, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/22250s017lbl.pdf
-
- Aubagio. Prescribing information. Sanofi; 2019. Accessed October 18, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202992s006lbl.pdf
-
- Epidiolex. Prescribing information. Greenwich Biosciences, Inc.; 2018. Accessed October 18, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous