Quantitative retrospective natural history modeling of WDR45-related developmental and epileptic encephalopathy - a systematic cross-sectional analysis of 160 published cases
- PMID: 34818117
- PMCID: PMC9298448
- DOI: 10.1080/15548627.2021.1990671
Quantitative retrospective natural history modeling of WDR45-related developmental and epileptic encephalopathy - a systematic cross-sectional analysis of 160 published cases
Abstract
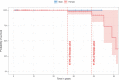
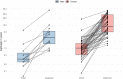
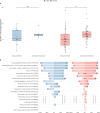
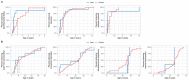
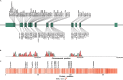
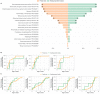
WDR45-related neurodevelopmental disorder (NDD) is a clinically-heterogenous congenital disorder of macroautophagy/autophagy. The natural history of this ultra-orphan disease remains incompletely understood, leading to delays in diagnosis and lack of quantifiable outcome measures. In this cross-sectional study, we model quantitative natural history data for WDR45-related NDD using a standardized analysis of 160 published cases, representing the largest cohort to date. The primary outcome of this study was survival. Age at disease onset, diagnostic delay and geographic distribution were quantified as secondary endpoints. Our tertiary aim was to explore and quantify the spectrum of WDR45-related phenotypes. Survival estimations showed low mortality until 39 years of age. Median age at onset was 10 months, with a median diagnostic delay of 6.2 years. Geographic distribution appeared worldwide with clusters in North America, East Asia, Western Europe and the Middle East. The clinical spectrum was highly variable with a bi-phasic evolution characterized by early-onset developmental and epileptic encephalopathy during childhood followed by a progressive dystonia-parkinsonism syndrome along with cognitive decline during early adulthood. Female individuals showed milder disease severity. The majority of pathogenic WDR45 variants were predicted to result in a loss of WDR45 expression, without clear genotype-phenotype associations. Our results provide clinical and epidemiological data that may facilitate an earlier diagnosis, enable anticipatory guidance and counseling of affected families and provide the foundation for endpoints for future interventional trials.Abbreviations: BPAN: beta-propeller protein-associated neurodegeneration; CNS: central nervous system; DEE: developmental and epileptic encephalopathy; MRI: magnetic resonance imaging; NBIA: neurodegeneration with brain iron accumulation; NDD: neurodevelopmental disorder; NGS: next-generation sequencing; WDR45/WIPI4: WD repeat domain 45.
Keywords: BPAN; Beta-propeller protein-associated neurodegeneration; DEE; NBIA; NBIA5; NDD; WDR45; WIPI4; congenital disorder of autophagy.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Prevalence of rare diseases: bibliographic data [Internet]. Orphanet Report Series, Rare Diseases collection, January 2021, Number 1: Diseases listed in alphabetical order; 2021. Available from: https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseas.... Accessed 9 Mar 2021.
-
- Haack TB, Hogarth P, Kruer MC, et al. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, x-linked dominant form of NBIA. Am J Hum Genet [Internet]. 2012 Dec 7;91(6):1144–1149. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23176820. Accessed 4 Dec 2020. - PMC - PubMed
-
- Saitsu H, Nishimura T, Muramatsu K, et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet [Internet]. 2013;45(4):445–449,449e1. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23435086. Accessed 4 Dec 2020. - PubMed
-
- Wan H, Wang Q, Chen X, et al. WDR45 contributes to neurodegeneration through regulation of ER homeostasis and neuronal death. Autophagy [Internet]. 2020 Mar;16(3):531–547. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31204559. Accessed 17 Feb 2021. - PMC - PubMed
-
- Ji C, Zhao H, Chen D, et al. Beta-propeller proteins WDR45 and WDR45B regulate autophagosome maturation into autolysosomes in neural cells. Curr Biol [Internet]. 2021 Feb;31(8)1666–1677.e6. Available from: https://www.ncbi.nlm.nih.gov/pubmed/33636118. Accessed 1 Mar 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
