Identification of Piezo1 channels in perivascular adipose tissue (PVAT) and their potential role in vascular function
- PMID: 34818570
- PMCID: PMC9301055
- DOI: 10.1016/j.phrs.2021.105995
Identification of Piezo1 channels in perivascular adipose tissue (PVAT) and their potential role in vascular function
Abstract
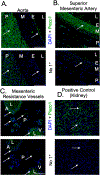
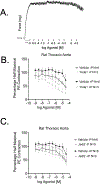
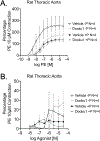
The vasculature constantly experiences distension/pressure exerted by blood flow and responds to maintain homeostasis. We hypothesized that activation of the stretch sensitive, non-selective cation channel Piezo1 would directly increase vascular contraction in a way that might be modified by perivascular adipose tissue (PVAT). The presence and function of Piezo1 was investigated by RT-PCR, immunohistochemistry, and isolated tissue bath contractility. Superior and mesenteric resistance arteries, aortae, and their PVATs from male Sprague Dawley rats were used. Piezo1 mRNA was detected in aortic vessels, aortic PVAT, mesenteric vessels, and mesenteric PVAT. Both adipocytes and stromal vascular fraction of mesenteric PVAT expressed Piezo1 mRNA. In PVAT, expression of Piezo1 mRNA was greater in magnitude than that of Piezo2, transient receptor potential cation channel, subfamily V, member 4 (TRPV4), anoctamin 1, calcium activated chloride channel (TMEM16), and Pannexin1 (Panx1). Piezo1 protein was present in endothelium and PVAT of rat aortic and in PVAT of mesenteric artery. The Piezo1 agonists Yoda1 and Jedi2 (1 nM - 10 µM) did not stimulate aortic contraction [max < 10% phenylephrine (PE) 10 µM contraction] or relaxation in tissues + or -PVAT. Depolarizing the aorta by modestly elevated extracellular K+ did not unmask aortic contraction to Yoda1 (max <10% PE 10 µM contraction). Finally, the Piezo1 antagonist Dooku1 did not modify PE-induced aorta contraction + or -PVAT. Surprisingly, Dooku1 directly caused aortic contraction in the absence (Dooku1 =26 ± 11; Vehicle = 11 ± 11%PE contraction) but not in the presence of PVAT (Dooku1 = 2 ± 1; Vehicle = 8 ± 5% PE contraction). Thus, Piezo1 is present and functional in the isolated rat aorta but does not serve direct vascular contraction with or without PVAT. We reaffirmed the isolated mouse aorta relaxation to Yoda1, indicating a species difference in Piezo1 activity between mouse and rat.
Keywords: Acetylcholine (PubChem CID: 6060); Dooku1 (PubChem CID: 137321150); Endothelium; Jedi2 (PubChem CID: 2796026); PVAT; Phenylephrine (PubChem CID: 6041); Piezo; Vascular; Yoda1 (PubChem CID: 2746822).
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interest
The authors declare no conflict of interest.
Figures






References
-
- Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A, Honoré E, Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling, Cell Rep. 13 (6) (2015) 1161–1171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

