The N501Y spike substitution enhances SARS-CoV-2 infection and transmission
- PMID: 34818667
- PMCID: PMC8900207
- DOI: 10.1038/s41586-021-04245-0
The N501Y spike substitution enhances SARS-CoV-2 infection and transmission
Abstract
The B.1.1.7 variant (also known as Alpha) of SARS-CoV-2, the cause of the COVID-19 pandemic, emerged in the UK in the summer of 2020. The prevalence of this variant increased rapidly owing to an increase in infection and/or transmission efficiency1. The Alpha variant contains 19 nonsynonymous mutations across its viral genome, including 8 substitutions or deletions in the spike protein that interacts with cellular receptors to mediate infection and tropism. Here, using a reverse genetics approach, we show that of the 8 individual spike protein substitutions, only N501Y resulted in consistent fitness gains for replication in the upper airway in a hamster model as well as in primary human airway epithelial cells. The N501Y substitution recapitulated the enhanced viral transmission phenotype of the eight mutations in the Alpha spike protein, suggesting that it is a major determinant of the increased transmission of the Alpha variant. Mechanistically, the N501Y substitution increased the affinity of the viral spike protein for cellular receptors. As suggested by its convergent evolution in Brazil, South Africa and elsewhere2,3, our results indicate that N501Y substitution is an adaptive spike mutation of major concern.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures




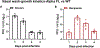







Update of
-
The N501Y spike substitution enhances SARS-CoV-2 transmission.bioRxiv [Preprint]. 2021 Mar 9:2021.03.08.434499. doi: 10.1101/2021.03.08.434499. bioRxiv. 2021. Update in: Nature. 2022 Feb;602(7896):294-299. doi: 10.1038/s41586-021-04245-0. PMID: 33758836 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

