Novel understanding on genetic mechanisms of enteric neuropathies leading to severe gut dysmotility
- PMID: 34818877
- PMCID: PMC8636838
- DOI: 10.4081/ejh.2021.3289
Novel understanding on genetic mechanisms of enteric neuropathies leading to severe gut dysmotility
Abstract
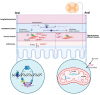
The enteric nervous system (ENS) is the third division of the autonomic autonomic nervous system and the largest collection of neurons outside the central nervous system (CNS). The ENS has been referred to as "the brain in the gut" or "the second brain of the human body" because of its highly integrated neural circuits controlling a vast repertoire of gut functions, including absorption/secretion, splanchnic blood vessels, some immunological aspects, intestinal epithelial barrier, and gastrointestinal (GI) motility. The latter function is the result of the ENS fine-tuning over smooth musculature, along with the contribution of other key cells, such as enteric glia (astrocyte like cells supporting and contributing to neuronal activity), interstitial cells of Cajal (the pacemaker cells of the GI tract involved in neuromuscular transmission), and enteroendocrine cells (releasing bioactive substances, which affect gut physiology). Any noxa insult perturbing the ENS complexity may determine a neuropathy with variable degree of neuro-muscular dysfunction. In this review, we aim to cover the most recent update on genetic mechanisms leading to enteric neuropathies ranging from Hirschsprung's disease (characterized by lack of any enteric neurons in the gut wall) up to more generalized form of dysmotility such as chronic intestinal pseudo-obstruction (CIPO) with a significant reduction of enteric neurons. In this line, we will discuss the role of the RAD21 mutation, which we have demonstrated in a family whose affected members exhibited severe gut dysmotility. Other genes contributing to gut motility abnormalities will also be presented. In conclusion, the knowledge on the molecular mechanisms involved in enteric neuropathy may unveil strategies to better manage patients with neurogenic gut dysmotility and pave the way to targeted therapies.
Figures

Similar articles
-
Enteric Neuromyopathies: Highlights on Genetic Mechanisms Underlying Chronic Intestinal Pseudo-Obstruction.Biomolecules. 2022 Dec 10;12(12):1849. doi: 10.3390/biom12121849. Biomolecules. 2022. PMID: 36551277 Free PMC article. Review.
-
Clinical and Pathological Features of Severe Gut Dysmotility.Adv Exp Med Biol. 2022;1383:9-17. doi: 10.1007/978-3-031-05843-1_2. Adv Exp Med Biol. 2022. PMID: 36587142
-
Mutations in RAD21 disrupt regulation of APOB in patients with chronic intestinal pseudo-obstruction.Gastroenterology. 2015 Apr;148(4):771-782.e11. doi: 10.1053/j.gastro.2014.12.034. Epub 2015 Jan 6. Gastroenterology. 2015. PMID: 25575569 Free PMC article.
-
Enteric neuron density correlates with clinical features of severe gut dysmotility.Am J Physiol Gastrointest Liver Physiol. 2019 Dec 1;317(6):G793-G801. doi: 10.1152/ajpgi.00199.2019. Epub 2019 Sep 23. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31545923 Free PMC article.
-
New insights into human enteric neuropathies.Neurogastroenterol Motil. 2004 Apr;16 Suppl 1:143-7. doi: 10.1111/j.1743-3150.2004.00491.x. Neurogastroenterol Motil. 2004. PMID: 15066021 Review.
Cited by
-
Multi-disciplinary Insights from the First European Forum on Visceral Myopathy 2022 Meeting.Dig Dis Sci. 2023 Oct;68(10):3857-3871. doi: 10.1007/s10620-023-08066-1. Epub 2023 Aug 31. Dig Dis Sci. 2023. PMID: 37650948 Free PMC article. Review.
-
Probiotics and microbial metabolites maintain barrier and neuromuscular functions and clean protein aggregation to delay disease progression in TDP43 mutation mice.Gut Microbes. 2024 Jan-Dec;16(1):2363880. doi: 10.1080/19490976.2024.2363880. Epub 2024 Jun 11. Gut Microbes. 2024. PMID: 38860943 Free PMC article.
-
Conditional deletion of IP3R1 by Islet1-Cre in mice reveals a critical role of IP3R1 in interstitial cells of Cajal in regulating GI motility.J Gastroenterol. 2025 Feb;60(2):152-165. doi: 10.1007/s00535-024-02164-1. Epub 2024 Oct 30. J Gastroenterol. 2025. PMID: 39476178
-
Enteric Neuromyopathies: Highlights on Genetic Mechanisms Underlying Chronic Intestinal Pseudo-Obstruction.Biomolecules. 2022 Dec 10;12(12):1849. doi: 10.3390/biom12121849. Biomolecules. 2022. PMID: 36551277 Free PMC article. Review.
-
Electroacupuncture Alleviates Functional Constipation in Mice by Activating Enteric Glial Cell Autophagy via PI3K/AKT/mTOR Signaling.Chin J Integr Med. 2023 May;29(5):459-469. doi: 10.1007/s11655-023-3594-3. Epub 2023 Mar 28. Chin J Integr Med. 2023. PMID: 36973529
References
-
- Furness J.B. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 2012;9:286-94. - PubMed
-
- Sternini C, Wong H, Pham T, De Giorgio R, Miller LJ, Kuntz SM, et al. . Expression of cholecystokinin a receptors in neurons innervating the rat stomach and intestine. Gastroenterology 1999;117:1136-46. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

