Efficacy and safety of fremanezumab in clinical trial participants aged ≥60 years with episodic or chronic migraine: pooled results from 3 randomized, double-blind, placebo-controlled phase 3 studies
- PMID: 34819017
- PMCID: PMC8903616
- DOI: 10.1186/s10194-021-01351-2
Efficacy and safety of fremanezumab in clinical trial participants aged ≥60 years with episodic or chronic migraine: pooled results from 3 randomized, double-blind, placebo-controlled phase 3 studies
Erratum in
-
Correction: Efficacy and safety of fremanezumab in clinical trial participants aged ≥60 years with episodic or chronic migraine: pooled results from 3 randomized, double-blind, placebo-controlled phase 3 studies.J Headache Pain. 2022 May 17;23(1):57. doi: 10.1186/s10194-022-01423-x. J Headache Pain. 2022. PMID: 35581545 Free PMC article. No abstract available.
Abstract
Background: Although migraine is less common in older people, preventive treatment of migraine in these individuals may be more challenging due to the presence of multiple comorbidities and polypharmacy. Additionally, evidence for migraine treatment efficacy, safety, and tolerability is limited in this population. We evaluated efficacy, safety, and tolerability of fremanezumab, a fully humanized monoclonal antibody (IgG2Δa) that selectively targets calcitonin gene-related peptide (CGRP), in clinical trial participants aged ≥60 years with episodic migraine (EM) or chronic migraine (CM).
Methods: This analysis included data from 3 randomized, double-blind, placebo-controlled phase 3 studies: the HALO EM study, HALO CM study, and FOCUS study in participants with EM or CM and prior inadequate response to 2-4 migraine preventive medication classes. Participants in all studies were randomized 1:1:1 to receive 12 weeks of subcutaneous treatment with quarterly fremanezumab (Months 1/2/3: EM/CM, 675 mg/placebo/placebo), monthly fremanezumab (Months 1/2/3: EM, 225 mg/225 mg/225 mg; CM, 675 mg/225 mg/225 mg), or matched monthly placebo.
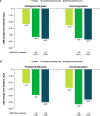
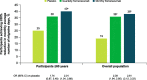
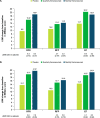
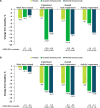
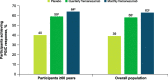
Results: These pooled analyses included 246 participants aged ≥60 years. Reductions in monthly migraine days from baseline over 12 weeks were significantly greater with fremanezumab (least-squares mean change from baseline [standard error]: quarterly fremanezumab, - 4.3 [0.59]; monthly fremanezumab, - 4.6 [0.54]) versus placebo (placebo, - 2.3 [0.57]; both P < 0.01 vs placebo). As early as Week 1, significant reductions from baseline in weekly migraine days were observed with fremanezumab versus placebo (both P < 0.01). With fremanezumab treatment versus placebo, a significantly higher proportion of participants achieved ≥50% reduction in monthly migraine days, and significant improvements in disability and quality-of-life outcomes were observed (P < 0.05). Proportions of participants experiencing serious adverse events and adverse events leading to discontinuation were low and similar in the fremanezumab and placebo groups. Efficacy and safety results were comparable to the overall pooled population (N = 2843).
Conclusions: This pooled subgroup analysis demonstrates that fremanezumab treatment is efficacious and well-tolerated over 12 weeks in participants aged ≥60 years with EM or CM. These data may help healthcare providers with clinical decision making and preventive treatment selection for older patients with migraine.
Trial registration: ClinicalTrials.gov identifiers: HALO CM: NCT02621931 ; HALO EM: NCT02629861 ; FOCUS: NCT03308968 .
Keywords: CGRP; Chronic migraine; Episodic migraine; Fremanezumab; Older age.
© 2021. The Author(s).
Conflict of interest statement
S.J.N. has received honoraria for consulting from Allergan/AbbVie, Amgen/Novartis, Axsome, BioDelivery Sciences, Biohaven, Eli Lilly, Fenix Group International, Impel, Nesos Corp (formerly Vorso Corp), and Teva Pharmaceuticals. S.J.N. has received honoraria for speaking from Allergan/AbbVie, Amgen/Novartis, Eli Lilly, and Teva Pharmaceuticals. S.J.N. has received honoraria for work in education or publishing from the American Academy of Neurology, the American Headache Society, Evolve Med Ed, the Massachusetts Medical Society, MedLink Neurology, MJH Life Sciences, NACCME, Neurology Learning Network, Pennsylvania Neurologic Society, Springer, WebMD/Medscape, and Wolters-Kluwer. S.J.N. has received legal fees for serving as a medical expert to Jackson & Campbell. J.M.C. is a former employee of Teva Pharmaceuticals. X.N., L.J., V.R.C., and L.J.K. are employees of Teva Pharmaceuticals. D.H.-L. has no competing interests. D.K. has served as an advisor to Alder, Amgen, and Eli Lilly and has received research support from Alder, Allergan, Amgen, CoLucid-Eli Lilly, Genentech, Teva Pharmaceuticals, VM Biopharma, and Zosano. C.L. has received personal fees from Allergan, Eli Lilly, Novartis, and Teva Pharmaceuticals; has participated in clinical trials as a principal investigator for Novartis and Teva Pharmaceuticals; and has no ownership interest and does not own stocks in any pharmaceutical company.
Figures




References
-
- Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, on behalf of Lifting the Burden: the Global Campaign Against Headache Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. doi: 10.1186/s10194-020-01208-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

