Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study
- PMID: 34819275
- PMCID: PMC9083235
- DOI: 10.1136/bmj-2021-067873
Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study
Abstract
Objectives: To determine whether time elapsed since the second injection of the Pfizer-BioNTech BNT162b2 mRNA vaccine was significantly associated with the risk of covid-19 infection after vaccination in people who received two vaccine injections.
Design: Test negative design study.
Setting: Electronic health records of a large state mandated healthcare organisation, Israel.
Participants: Adults aged ≥18 years who had received a reverse transcription polymerase chain reaction (RT-PCR) test between 15 May 2021 and 17 September 2021, at least three weeks after their second vaccine injection, had not received a third vaccine injection, and had no history of covid-19 infection.
Main outcome measures: Positive result for the RT-PCR test. Individuals who tested positive for SARS-CoV-2 and controls were matched for week of testing, age category, and demographic group (ultra-orthodox Jews, individuals of Arab ancestry, and the general population). Conditional logistic regression was adjusted for age, sex, socioeconomic status, and comorbid conditions.
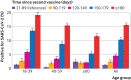
Results: 83 057 adults received an RT-PCR test for SARS-CoV-2 during the study period and 9.6% had a positive result. Time elapsed since the vaccine injection was significantly longer in individuals who tested positive (P<0.001). Adjusted odds ratio for infection at time intervals >90 days since vaccination were significantly increased compared with the reference of <90 days: 2.37 (95% confidence interval 1.67 to 3.36) for 90-119 days, 2.66 (1.94 to 3.66) for 120-149 days, 2.82 (2.07 to 3.84) for 150-179 days, and 2.82 (2.07 to 3.85) for ≥180 days (P<0.001 for each 30 day interval).
Conclusions: In this large population of adults tested for SARS-CoV-2 by RT-PCR after two doses of mRNA BNT162b2 vaccine, a gradual increase in the risk of infection was seen for individuals who received their second vaccine dose after at least 90 days.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Leumit Health Services and the Intramural Research Program, National Institutes of Health, National Cancer Institute, Centre for Cancer Research for the submitted work; the authors declare no competing interests; AI, EMe, AAS, YS, IG, AG-C, EMa,and SV are employees of Leumit Health Services; all authors declare that they have no other relationships or activities that could appear to have influenced the submitted work.
Figures
Update of
-
Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort.medRxiv [Preprint]. 2021 Aug 5:2021.08.03.21261496. doi: 10.1101/2021.08.03.21261496. medRxiv. 2021. Update in: BMJ. 2021 Nov 24;375:e067873. doi: 10.1136/bmj-2021-067873. PMID: 34401882 Free PMC article. Updated. Preprint.
Comment in
-
Covid-19 vaccines, immunity, and boosters.BMJ. 2021 Dec 20;375:n3105. doi: 10.1136/bmj.n3105. BMJ. 2021. PMID: 34930779 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous