Trpm5 channels encode bistability of spinal motoneurons and ensure motor control of hindlimbs in mice
- PMID: 34819493
- PMCID: PMC8613399
- DOI: 10.1038/s41467-021-27113-x
Trpm5 channels encode bistability of spinal motoneurons and ensure motor control of hindlimbs in mice
Abstract
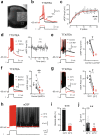
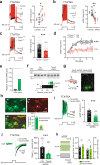
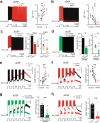
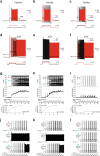
Bistable motoneurons of the spinal cord exhibit warmth-activated plateau potential driven by Na+ and triggered by a brief excitation. The thermoregulating molecular mechanisms of bistability and their role in motor functions remain unknown. Here, we identify thermosensitive Na+-permeable Trpm5 channels as the main molecular players for bistability in mouse motoneurons. Pharmacological, genetic or computational inhibition of Trpm5 occlude bistable-related properties (slow afterdepolarization, windup, plateau potentials) and reduce spinal locomotor outputs while central pattern generators for locomotion operate normally. At cellular level, Trpm5 is activated by a ryanodine-mediated Ca2+ release and turned off by Ca2+ reuptake through the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump. Mice in which Trpm5 is genetically silenced in most lumbar motoneurons develop hindlimb paresis and show difficulties in executing high-demanding locomotor tasks. Overall, by encoding bistability in motoneurons, Trpm5 appears indispensable for producing a postural tone in hindlimbs and amplifying the locomotor output.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Persistent Nav1.1 and Nav1.6 currents drive spinal locomotor functions through nonlinear dynamics.Cell Rep. 2023 Sep 26;42(9):113085. doi: 10.1016/j.celrep.2023.113085. Epub 2023 Sep 3. Cell Rep. 2023. PMID: 37665666
-
Effect of size on expression of bistability in mouse spinal motoneurons.J Neurophysiol. 2024 Apr 1;131(4):577-588. doi: 10.1152/jn.00320.2023. Epub 2024 Feb 21. J Neurophysiol. 2024. PMID: 38380829 Free PMC article.
-
Whole cell recordings of lumbar motoneurons during locomotor-like activity in the in vitro neonatal rat spinal cord.J Neurophysiol. 1998 Feb;79(2):743-52. doi: 10.1152/jn.1998.79.2.743. J Neurophysiol. 1998. PMID: 9463437
-
Ascending pathways that mediate cholinergic modulation of lumbar motor activity.J Neurochem. 2017 Aug;142 Suppl 2:82-89. doi: 10.1111/jnc.14065. J Neurochem. 2017. PMID: 28791705 Review.
-
Contribution of postural muscle tone to full expression of posture and locomotor movements: multi-faceted analyses of its setting brainstem-spinal cord mechanisms in the cat.Jpn J Physiol. 1989;39(6):785-809. Jpn J Physiol. 1989. PMID: 2698966 Review.
Cited by
-
Multistability of bursting rhythms in a half-center oscillator and the protective effects of synaptic inhibition.Front Cell Neurosci. 2024 Sep 17;18:1395026. doi: 10.3389/fncel.2024.1395026. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39355175 Free PMC article.
-
A depression-associated protein FKBP5 functions in autophagy initiation through scaffolding the VPS34 complex.Mol Neurobiol. 2025 Aug;62(8):9916-9934. doi: 10.1007/s12035-025-04897-3. Epub 2025 Apr 2. Mol Neurobiol. 2025. PMID: 40175715
-
Voluntary activation of muscle in humans: does serotonergic neuromodulation matter?J Physiol. 2022 Aug;600(16):3657-3670. doi: 10.1113/JP282565. Epub 2022 Aug 1. J Physiol. 2022. PMID: 35864781 Free PMC article. Review.
-
Intrinsic motoneuron properties in typical human development.J Physiol. 2024 May;602(9):2061-2087. doi: 10.1113/JP285756. Epub 2024 Mar 30. J Physiol. 2024. PMID: 38554126 Free PMC article.
-
Ionic Mechanisms Underlying Bistability in Spinal Motoneurons: Insights from a Computational Model.bioRxiv [Preprint]. 2025 Jun 10:2025.06.06.658369. doi: 10.1101/2025.06.06.658369. bioRxiv. 2025. PMID: 40661631 Free PMC article. Preprint.
References
-
- Kiehn O. Plateau potentials and active integration in the ‘final common pathway’ for motor behaviour. Trends Neurosci. 1991;14:68–73. - PubMed
-
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog. Brain Res. 1999;123:39–48. - PubMed
-
- Brownstone RM, Gossard JP, Hultborn H. Voltage-dependent excitation of motoneurones from spinal locomotor centres in the cat. Exp. Brain Res. 1994;102:34–44. - PubMed
-
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog. Brain Res. 2004;143:77–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

