Diagnostic performance of rapid antigen tests (RATs) for SARS-CoV-2 and their efficacy in monitoring the infectiousness of COVID-19 patients
- PMID: 34819567
- PMCID: PMC8613285
- DOI: 10.1038/s41598-021-02197-z
Diagnostic performance of rapid antigen tests (RATs) for SARS-CoV-2 and their efficacy in monitoring the infectiousness of COVID-19 patients
Abstract
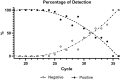
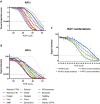
The most widely used test for the diagnosis of SARS-CoV-2 infection is a PCR test. PCR has very high sensitivity and is able to detect very low amounts of RNA. However, many individuals receiving a positive test result in a context of a PCR-based surveillance might be infected with SARS-CoV-2, but they are not contagious at the time of the test. The question arises regards if the cost effective, portable rapid antigen tests (RATs) have a better performance than PCR in identification of infectious individuals. In this direction, we examined the diagnostic performance of RATs from 14 different manufacturers in 400 clinical samples with known rRT-PCR cycles threshold (cT) and 50 control samples. Substantial variability was observed in the limit of detection (LOD) of different RATs (cT = 26.8-34.7). The fluorescence-based RAT exhibited a LOD of cT = 34.7. The use of the most effective RATs leads to true positive rates (sensitivities) of 99.1% and 90.9% for samples with cT ≤ 30 and cT ≤ 33, respectively, percentages that can guarantee a sensitivity high enough to identify contagious patients. RAT testing may also substantially reduce the quarantine period for infected individuals without compromising personal or public safety.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Mandavilli, A. Your Coronavirus Test Is Positive. Maybe It Shouldn’t Be.https://www.nytimes.com/2020/08/29/health/coronavirus-testing.html(2020) (Accessed 11 November 2021).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

