The Immunomodulatory Functions of Butyrate
- PMID: 34819742
- PMCID: PMC8608412
- DOI: 10.2147/JIR.S300989
The Immunomodulatory Functions of Butyrate
Abstract
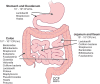
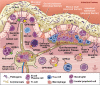
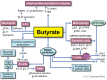
The gastrointestinal (GI) system contains many different types of immune cells, making it a key immune organ system in the human body. In the last decade, our knowledge has substantially expanded regarding our understanding of the gut microbiome and its complex interaction with the gut immune system. Short chain fatty acids (SCFA), and specifically butyrate, play an important role in mediating the effects of the gut microbiome on local and systemic immunity. Gut microbial alterations and depletion of luminal butyrate have been well documented in the literature for a number of systemic and GI inflammatory disorders. Although a substantial knowledge gap exists requiring the need for further investigations to determine cause and effect, there is heightened interest in developing immunomodulatory therapies by means of reprogramming of gut microbiome or by supplementing its beneficial metabolites, such as butyrate. In the current review, we discuss the role of endogenous butyrate in the inflammatory response and maintaining immune homeostasis within the intestine. We also present the experimental models and human studies which explore therapeutic potential of butyrate supplementation in inflammatory conditions associated with butyrate depletion.
Keywords: adaptive immunity; butyrate; dysbiosis; epithelial barrier; gut microbiome; gut microbiota; immunity; inflammation; inflammatory bowel disease; innate immunity; short chain fatty acids.
© 2021 Siddiqui and Cresci.
Conflict of interest statement
This work was supported by a National Institutes of Health grant (no. R01AA028043- 01A1 (Cresci) and 2T32DK083251-11A1 (MPI). The authors report no conflicts of interest in this work.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

