Chiral Phosphoric Acids as Versatile Tools for Organocatalytic Asymmetric Transfer Hydrogenations
- PMID: 34819797
- PMCID: PMC8597106
- DOI: 10.1002/ejoc.202100894
Chiral Phosphoric Acids as Versatile Tools for Organocatalytic Asymmetric Transfer Hydrogenations
Abstract
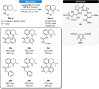
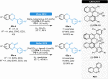
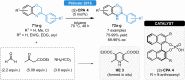
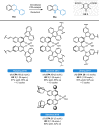
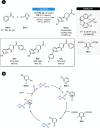
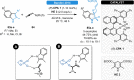
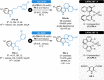
Herein, recent developments in the field of organocatalytic asymmetric transfer hydrogenation (ATH) of C=N, C=O and C=C double bonds using chiral phosphoric acid catalysis are reviewed. This still rapidly growing area of asymmetric catalysis relies on metal-free catalysts in combination with biomimetic hydrogen sources. Chiral phosphoric acids have proven to be extremely versatile tools in this area, providing highly active and enantioselective alternatives for the asymmetric reduction of α,β-unsaturated carbonyl compounds, imines and various heterocycles. Eventually, such transformations are more and more often used in multicomponent/cascade reactions, which undoubtedly shows their great synthetic potential and the bright future of organocatalytic asymmetric transfer hydrogenations.
Keywords: Asymmetric Transfer Hydrogenation; Catalysis; Chiral Phosphoric Acids; Metal-Free Hydrogenation; Organocatalysis.
© 2021 The Authors. European Journal of Organic Chemistry published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






















Similar articles
-
Brønsted-acid-catalyzed asymmetric multicomponent reactions for the facile synthesis of highly enantioenriched structurally diverse nitrogenous heterocycles.Acc Chem Res. 2011 Nov 15;44(11):1156-71. doi: 10.1021/ar2000343. Epub 2011 Jul 29. Acc Chem Res. 2011. PMID: 21800828
-
Counterion Enhanced Organocatalysis: A Novel Approach for the Asymmetric Transfer Hydrogenation of Enones.ChemCatChem. 2020 Jul 21;12(14):3776-3782. doi: 10.1002/cctc.202000414. Epub 2020 Jun 15. ChemCatChem. 2020. PMID: 32999691 Free PMC article.
-
Enantioselective organocatalytic transfer hydrogenation reactions using Hantzsch esters.Acc Chem Res. 2007 Dec;40(12):1327-39. doi: 10.1021/ar7001864. Acc Chem Res. 2007. PMID: 18085748 Review.
-
Construction of Axially Chiral Compounds via Asymmetric Organocatalysis.Acc Chem Res. 2018 Feb 20;51(2):534-547. doi: 10.1021/acs.accounts.7b00602. Epub 2018 Feb 8. Acc Chem Res. 2018. PMID: 29419282
-
Chiral Brønsted acids for asymmetric organocatalysis.Top Curr Chem. 2010;291:395-456. doi: 10.1007/978-3-642-02815-1_1. Top Curr Chem. 2010. PMID: 21494945 Review.
Cited by
-
A Telescoped Continuous Flow Enantioselective Process for Accessing Intermediates of 1-Aryl-1,3-diols as Chiral Building Blocks.J Org Chem. 2023 Nov 3;88(21):15523-15529. doi: 10.1021/acs.joc.3c02040. Epub 2023 Oct 16. J Org Chem. 2023. PMID: 37844195 Free PMC article.
-
Efficient construction of a β-naphthol library under continuous flow conditions.RSC Adv. 2024 Jan 15;14(4):2673-2677. doi: 10.1039/d3ra08660g. eCollection 2024 Jan 10. RSC Adv. 2024. PMID: 38226147 Free PMC article.
-
Total Synthesis and Stereochemical Assignment of Enteropeptin A.J Am Chem Soc. 2024 Jul 3;146(26):17629-17635. doi: 10.1021/jacs.4c06126. Epub 2024 Jun 23. J Am Chem Soc. 2024. PMID: 38909357 Free PMC article.
-
Catalytic Asymmetric Ionic Hydrogenation of α-Alkyl Styrenes.J Am Chem Soc. 2025 Sep 3;147(35):31463-31469. doi: 10.1021/jacs.5c11514. Epub 2025 Aug 19. J Am Chem Soc. 2025. PMID: 40831225 Free PMC article.
-
Broadly Applicable Copper(I)-Catalyzed Alkyne Semihydrogenation and Hydrogenation of α,β-Unsaturated Amides Enabled by Bifunctional Iminopyridine Ligands.J Am Chem Soc. 2025 Apr 30;147(17):14481-14490. doi: 10.1021/jacs.5c01339. Epub 2025 Apr 16. J Am Chem Soc. 2025. PMID: 40239054 Free PMC article.
References
-
- Ojima I., Catalytic Asymmetric Synthesis, 3rd Edition, Wiley-VCH, Weinheim, 2010.
-
- Blaser H. U., Federsel H.-J., Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions, 2nd Edition, Wiley-VCH, Weinheim, 2010.
-
- Yang J. W., Hechavarria Fonseca M. T., List B., J. Am. Chem. Soc. 2005, 127, 15036–15037. - PubMed
-
- Huang Y., Walji A. M., Larsen C. H., MacMillan D. W. C., J. Am. Chem. Soc. 2005, 127, 15051–15053. - PubMed
-
- Uraguchi D., Terada M., J. Am. Chem. Soc. 2004, 126, 5356–5357. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
