Synthesis and Characterization of Cobalt NCN Pincer Complexes
- PMID: 34819799
- PMCID: PMC8596404
- DOI: 10.1002/ejic.202100643
Synthesis and Characterization of Cobalt NCN Pincer Complexes
Abstract
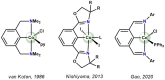
A series of cobalt complexes, stabilized by a monoanionic tridentate NCN pincer ligand, was synthetized and characterized. Preparation of the paramagnetic 15 VE complex [Co(NCNCH2-Et)Br] (1) was accomplished by transmetalation of Li[2,6-(Et2NCH2)2C6H3] with CoBr2 in THF. Treatment of this air-sensitive compound with NO gas resulted in the formation of the diamagnetic Co(III) species [Co(NCNCH2-Et)(NO)Br] (2) as confirmed by X-ray diffraction. This complex features a strongly bent NO ligand (Co-N-O∠135.0°). The νNO is observed at 1609 cm-1 which is typical for a bent metal-N-O arrangement. Coordinatively unsaturated 1 could further be treated with pyridine, isocyanides, phosphines and CO to form five-coordinate 17 VE complexes. Oxidation of 1 with CuBr2 led to the formation of the Co(III) complex [Co(NCNCH2-Et)Br2]. Treatment of [Co(NCNCH2-Et)Br2] with TlBF4 as halide scavenger in acetonitrile led to the formation of the cationic octahedral complex [Co(NCNCH2-Et)(MeCN)3](BF4)2. A combination of X-ray crystallography, IR-, NMR- and EPR-spectroscopy as well as DFT/CAS-SCF calculations were used to characterize all compounds.
Keywords: Cobalt; DFT studies; EPR studies; Nitrosyl ligand; Pincer complexes.
© 2021 The Authors. European Journal of Inorganic Chemistry published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






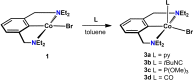

References
-
- For reviews on pincer complexes, see:
-
- Albrecht M., van Koten G., Angew. Chem. Int. Ed. 2001, 40, 3750–3781; - PubMed
- Angew. Chem. 2001, 113, 3866–3898;
-
- Valdos H., García-Eleno M. A., Canseco-Gonzalez D., Morales-Morales D., ChemCatChem 2018, 10, 3136–3172;
-
- Benito-Garagorri D., Kirchner K., Acc. Chem. Res. 2008, 41, 201–213; - PubMed
-
- Peris E., Crabtree R. H., Chem. Soc. Rev. 2018, 47, 1959–1968; - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
