Cerebral Venous Thrombosis: Clinical, Radiological, Biological, and Etiological Characteristics of a French Prospective Cohort (FPCCVT)-Comparison With ISCVT Cohort
- PMID: 34819911
- PMCID: PMC8606816
- DOI: 10.3389/fneur.2021.753110
Cerebral Venous Thrombosis: Clinical, Radiological, Biological, and Etiological Characteristics of a French Prospective Cohort (FPCCVT)-Comparison With ISCVT Cohort
Abstract
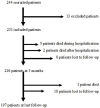
Introduction: Cerebral venous thrombosis (CVT) is a rare disease with highly variable clinical presentation and outcome. Etiological assessment may be negative. The clinical and radiological presentation and evolution can be highly variable. The mechanisms involved in this variability remain unknown. Objective: The aim of this multicenter French study registered on ClinicalTrials.gov (NCT02013635) was therefore to prospectively recruit a cohort of patients with cerebral venous thrombosis (FPCCVT) in order to study thrombin generation and clot degradation, and to evaluate their influence on clinical radiological characteristics. The first part of the study was to compare our cohort with a reference cohort. Methods: This prospective, multicenter, French study was conducted from July 2011 to September 2016. Consecutive patients (aged >15 years) referred to the stroke units of 21 French centers and who had a diagnosis of symptomatic CVT were included. All patients gave their written informed consent. The diagnosis of CVT had to be confirmed by imaging. Clinical, radiological, biological, and etiological characteristics were recorded at baseline, at acute phase, at 3 months and at last follow-up visit. Thrombophilia screening and the choice of treatment were performed by the attending physician. All data were compared with data from the International Study on CVT published by Ferro et al. Results: Two hundred thirty-one patients were included: 117 (50.6%) had isolated intracranial hypertension, 96 (41.5%) had focal syndrome. During hospitalization, 229 (99.1%) patients received anticoagulant treatment. Median length of hospital stay was 10 days. Five patients died during hospitalization (2.2%). At 3 months, 216 patients (97.0%) had follow-up with neurological data based on an outpatient visit. The mean duration of antithrombotic treatment was 9 months, and the mean time to last follow-up was 10.5 months. At the end of follow-up, eight patients had died, and 26 patients were lost to follow-up. At least one risk factor was identified in 200 patients. Conclusions: We demonstrated that the FPCCVT cohort had radiological, biological, and etiological characteristics similar to the historical ISCVT cohort. Nevertheless, the initial clinical presentation was less severe in our study probably due to an improvement in diagnostic methods between the two studies.
Keywords: French cohort; cerebral veins; prospective observational study; sinus; thrombosis.
Copyright © 2021 Triquenot Bagan, Crassard, Drouet, Barbieux-Guillot, Marlu, Robinet-Borgomino, Morange, Wolff, Grunebaum, Klapczynski, André-Kerneis, Pico, Martin-Bastenaire, Ellie, Menard, Rouanet, Freyburger, Godenèche, Allano, Moulin, Mourey, Derex, Berruyer, Runavot, Trichet, Viader, Le Querrec, Husein, Cluet-Dennetiere, Macian-Montoro, Donnard, Guillon, Ternisien, Zuber, Laplanche, Tassan, Peeltier, Canaple, Roussel, Gaillard, Scavazza and Le Cam Duchez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical