Crosstalk Between Intestinal Serotonergic System and Pattern Recognition Receptors on the Microbiota-Gut-Brain Axis
- PMID: 34819919
- PMCID: PMC8607755
- DOI: 10.3389/fendo.2021.748254
Crosstalk Between Intestinal Serotonergic System and Pattern Recognition Receptors on the Microbiota-Gut-Brain Axis
Abstract
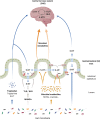
Disruption of the microbiota-gut-brain axis results in a wide range of pathologies that are affected, from the brain to the intestine. Gut hormones released by enteroendocrine cells to the gastrointestinal (GI) tract are important signaling molecules within this axis. In the search for the language that allows microbiota to communicate with the gut and the brain, serotonin seems to be the most important mediator. In recent years, serotonin has emerged as a key neurotransmitter in the gut-brain axis because it largely contributes to both GI and brain physiology. In addition, intestinal microbiota are crucial in serotonin signaling, which gives more relevance to the role of the serotonin as an important mediator in microbiota-host interactions. Despite the numerous investigations focused on the gut-brain axis and the pathologies associated, little is known regarding how serotonin can mediate in the microbiota-gut-brain axis. In this review, we will mainly discuss serotonergic system modulation by microbiota as a pathway of communication between intestinal microbes and the body on the microbiota-gut-brain axis, and we explore novel therapeutic approaches for GI diseases and mental disorders.
Keywords: 5-HT; NLRs; PRRs; TLRs; microorganisms; serotonin; tryptophan.
Copyright © 2021 Layunta, Buey, Mesonero and Latorre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

